
Patients with peripheral artery disease and depressive symptoms experience worse recovery than patients without depressive symptoms.

Patients with peripheral artery disease and depressive symptoms experience worse recovery than patients without depressive symptoms.

There is no greater support team available to students than their pharmacy school.

Effective time management can make the difference between a successful pharmacist and an unsuccessful one.
The declaration is in response to a May CDC report, which found a concerning decrease in routine childhood immunizations during the COVID-19 pandemic.
In an article in The BMJ, a panel of international experts presented recommendations regarding the use of remdesivir in patients with severe COVID-19, despite little evidence to support such a decision.
In a recent survey, just 36% of respondents indicated they will get the COVID-19 vaccination as soon as it is available.

The coronavirus disease 2019 pandemic has presented pharmacy students with an opportunity to develop new skills that former students never had the opportunity to develop.
New research suggests it is possible to treat cognitive decline with personalized, precision medicine.
Extended glucocorticoid use can cause complications such as cardiovascular disorders, infections, and osteoporosis, according to a new study.
Walgreens is now offering flu shots daily at all of its nearly 9100 pharmacies, with additional safety measures in place that follow the CDC’s recommendations for anyone over 6 months of age to get the flu shot.

Although the majority of survey respondents will continue using telehealth, providers must ensure the safety of their software in order to maintain patient trust.

Disparities in COVID-19 mortality may be caused by challenges in accessing health care and other resources, as well as socioeconomic factors that could increase exposure.

With millions of users between 13 and 24 years of age, TikTok offers a chance to educate those who could easily spread COVID-19 to older adults.

More than 10 million adults over age 50 have osteoporosis.

Flu vaccination will be particularly important in the 2020-2021 season to lessen the burden on the health care system and ensure adequate resources amid the coronavirus disease 2019 pandemic.
Despite, antiretroviral therapy, HIV can hide in blood and tissue.

Continuous glucose monitoring has revolutionized the way patients with diabetes can monitor their glucose.
SalivaDirect, developed by researchers at the Yale School of Public Health, is currently in use by the National Basketball Association to test players and staff for COVID-19.
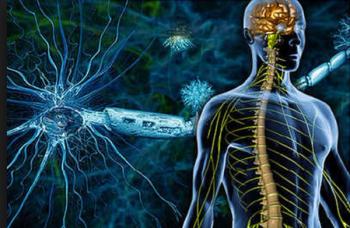
Fingolimod (Gilenya), which is approved by the FDA to treat multiple sclerosis flare-ups, may also block HIV and reduce the latent reservoir.

Pharmacy Times® interviewed Anne Marie Kondic, PharmD, the executive director of the Community Pharmacy Foundation (CPF), on the work CPF has been engaged in to promote the value of the practice of the community pharmacy.

Now, more than ever, is the time that nurses, pharmacists, pharmacy technicians, and physicians can help those who need access to life-saving medicines, and Dispensary of Hope is a resource.

As misconceptions surround some OTC supplements, changes to the diet may be beneficial in supporting brain health in populations experiencing age-related memory loss.

A patient has unexplained symptoms that appear to be severe anxiety.

Unique model demonstrates how clinical pharmacy services could be integrated into an interprofessional telehealth clinic.
Researchers attempt to address a lack of evidence showing that schools have a role in the transmission of COVID-19.

Leaders of Operation Warp Speed optimistic for a coronavirus disease 2019 vaccine following multi-billion dollar investments in several candidates.
NACDS states that although prescription drug discount cards were put in place to reduce out-of-pocket costs, states have been using the data in a manner inconsistent with federal law and policy.

Analysis suggests that the current diabetes screening criteria, despite covering about a quarter of US children and adolescents, do not capture many youth with hyperglycemia.
Pharmacy Times® interviewed Amanda Bowles, the director of the Shopper Science Lab at GSK Consumer Healthcare, on the expansion of coverage eligibility for OTC and menstrual products by health savings accounts and flexible spending accounts this year following the passage of the CARES Act.

The 2020 NCPA Annual Convention will be held exclusively online October 18-19, 2020.