
Precise, more potent therapy can offer reduced toxicity.

Precise, more potent therapy can offer reduced toxicity.

Women are 70% more likely to survive stage 1 breast cancer than stage 4, and new research shows that earlier screening could catch breast cancer earlier, increasing their survival rate.
Long-term follow up on the KEYNOTE-604 study suggests pembrolizumab plus etoposide/platinum is a superior first-line therapy for small cell lung cancer vs placebo.

Irinotecan liposomal injection (Onivyde) used as second-line therapy for patients with small cell lung cancer did not meet the primary end point of overall survival compared to topotecan.

Building a team to support best practices, medication safety, and staff education can reduce risks.

New drugs, virtual care, and a patient-focused approach are key during this period of transformation.

Andrew Barnell, MBA, CEO and co-founder of Geneoscopy, discusses his meeting at the White House on President Biden’s Cancer Moonshot initiative.
Professionals address key challenges including structural racism and the hematology-oncology pharmacist great migration.
The treatment regimen is first in more than 20 years to significantly improve outcomes in individuals with the fast-growing cancer, according to the company.

Researchers suggest that immune checkpoint inhibitors significantly preserve the quality of life in patients with cancer.
For pediatric patients with B-cell acute lymphoblastic leukemia, higher doses of tisagenlecleucel increased their rate of survival after 1 year by nearly 30%.
The drug also meets the key secondary endpoint of improved overall survival for treatment for individuals with HER2 positive unresectable and/or metastatic breast cancer.

Zynteglo was approved for the treatment of adult and pediatric patients with beta-thalassemia who require regular red blood cell transfusions after safety and efficacy were demonstrated in clinical trials.
As local drug delivery technology evolves, the field is set for monumental change.

FDA approved dexamethasone tablets USP 1.5mg, 4mg and 6mg, providing a new generic alternative to the steroid Decadron.

Gilead Sciences Inc submitted a supplemental Biologics License Application to the FDA for sacituzumab govitecan-hziy (Trodelvy) following promising trial results.
Scientists found that inhibiting certain immune system receptors could treat colon cancer and other diseases of the gastrointestinal tract.

The Oncomine Dx Target Test is a next-generation sequencing–based companion diagnostic developed to analyze 23 genes associated with non–small cell lung cancer in patients who harbor an activating HER2 mutation.

A new study found that reduced tobacco use lowered deaths, person-years of lost life, and lost earnings across many states nationwide.
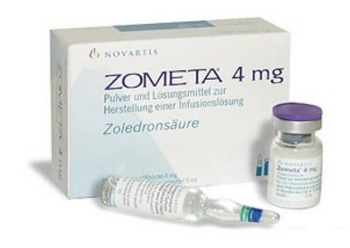
Zoledronic acid (Zometa) is indicated for the treatment of hypercalcemia that may occur with cancer.
Researchers discovered that a common anti-inflammatory drug could lower the number of patients on ventilators from COVID-19, increasing the likelihood of survival by more than 50%.

Both ibrutinib (Imbruvica) and venetoclax (Venclexta) carry an approved indication for use in chronic lymphocytic leukemia but do not often lead to complete remission, and therapy routinely continues indefinitely or until disease progression.

Specialty pharmacies are well positioned to address disparities that affect outcomes.
Although some physicians caution against menopausal hormone therapy, a new study finds that it does not lead to breast cancer recurrence or mortality.

As treatments improve and prostate cancer potentially becomes more of a chronic illness, pharmacists have a chance to shine.

Study finds link between higher insulin doses and the risk of developing cancer in patients with type 1 diabetes.
The FDA approval of fam-trastuzumab deruxtecan-nxki (Enhertu) to treat HER2 mutant non-small cell lung cancer was based on results from the DESTINY-Lung02 phase 2 trial.
New study shows that race does not influence outcomes from immunotherapy for aggressive triple-negative breast cancer.
The interim analysis of the TROPION-Lung02 trial demonstrate an overall response rate of 37% in individuals who were treated with datopotamab deruxtecan and pembrolizumab.

Last year, the majority of new drug approvals were for small molecules, including treatments for HIV, cancer, infections, heart and kidney disease, and neurological disorders.