
Expert explains how delaying BCOP certification too long after a PGY2 residency can cause a “if you don’t use it, you’ll lose it” problem.

Expert explains how delaying BCOP certification too long after a PGY2 residency can cause a “if you don’t use it, you’ll lose it” problem.

CAR T therapies are potentially revolutionizing the treatment of acute lymphoblastic leukemia (ALL).

The annual report notes that progress in reducing cancer mortality is uneven among populations, with minority groups not seeing the same benefits from therapeutic advances.

Danielle Carnival, PhD, addressed the National Comprehensive Cancer Network Policy Summit on Friday.
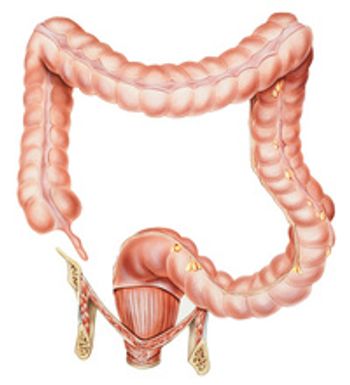
The FDA accepted a supplemental New Drug Application for tucatinib to treat HER2 colorectal cancer, which could significantly improve outcomes for these patients.

The All of Us Research Program looks to enroll more than 1 million US individuals in the next 10 years to enable public access to medical data for research purposes.

Selpercatinib produced clinically meaningful and durable responses across a variety of tumor types in patients with RET-driven cancers.

Data showed that Black women, regardless of education level or income, are more likely to reside in disadvantaged neighborhoods, which may contribute to racial disparities in breast cancer.
Nivolumab is a biomarker that has been shown to benefit the survival of patients with stage 2 melanoma patients compared with placebo.

Second- and third-generation individuals living in Los Angeles, California, demonstrate 35% and 61%, respectively, more of a chance of getting the disease, according to new study results.

Black women are suggested to be diagnosed with stage 3 HR-positive breast cancer and receive longer neoadjuvant endocrine therapy more often than White women.
Phase 3 TOPAZ-1 trial of AstraZeneca’s durvalumab demonstrates an improved overall survival benefit.
AstraZeneca’s Lynparza in combination or as a monotherapy demonstrates meaningful survival benefit in long-term follow-up.
Study indicates that a diet high in ultra-processed food is more likely to increase the risk of colorectal cancer in men than it is women.

Analysis shows that individuals who are comfortable sharing their identities with providers are more likely to be satisfied with overall care.

Management of marginal zone lymphoma was impacted by the COVID-19 pandemic because some treatment options can reduce B-cell-produced antibodies.

NVG-111 is a humanized, tandem single-chain variable fragment ROR1 x CD3 BiTE being evaluated in relapsed/refractory chronic lymphocytic leukemia and mantle cell lymphoma.

Eflapegrastism-xnst injection (Rolvedon, Spectrum Pharmaceuticals) is indicated to lower the incidence of infection, as demonstrated by febrile neutropenia, in adult patients with non-myeloid malignancies.

Researchers found differences in the variable importance of cancer mortality risk factors depending on an individual’s geographical region.

Prior authorizations have delayed time to treatment for patients and can directly impact clinical outcomes.
Triplet combination demonstrates a reduction in the risk of disease progression or death in individuals with previously untreated advanced intermediate- or poor-risk renal cell carcinoma.
Therapy shows positive clinical efficacy and favorable tolerability as a monotherapy and in combination with cetuximab in heavily pretreated patients who have a KRASG12C mutation.
New research presented at ESMO Congress found that certain patients with advanced melanoma administered a combination of cemiplimab with fianlimab experienced a median progression-free survival of 2 years.
Researchers at the European Society for Medical Oncology Congress presented findings that show osimertinib may clinically reduce the risk of lung cancer recurrence.

Research suggests that rucaparib can increase progression-free survival by more than a year in women with advanced ovarian cancer.

Trastuzumab deruxtecan produces a confirmed objective response rate of 53.8% and 42.9% in the 5.4 mg/kg arm and 6.4 mg/kg treatment arms, respectively, among patients with HER2 mutant non-small cell lung cancer.

An estimated 31.4% of individuals with non-squamous non–small cell lung cancer treated with the combination were alive at 3 years compared to 17.3% for those on chemotherapy alone.

Results demonstrated a 64.5% confirmed objective response rate in patients treated with the investigational combination.

Ribociclib with endocrine therapy was found to increase the median overall survival of patients with HR+/HER2- advanced breast cancer with visceral metastases to nearly 5 years.
Data presented at the 2022 ESMO congress show poziotinib has high activity in both treatment-naïve and previously treated patients with non-small cell lung cancer.