The updated efficacy analyses showed that tislelizumab in combination with chemotherapy continued to demonstrate a clinically significant progression-free survival benefit for individuals with recurrent or metastatic nasopharyngeal cancer.

The updated efficacy analyses showed that tislelizumab in combination with chemotherapy continued to demonstrate a clinically significant progression-free survival benefit for individuals with recurrent or metastatic nasopharyngeal cancer.
The analyses are shared at the American Association for Cancer Research Annual Meeting 2022 in New Orleans, Louisiana.
Analysis indicates that quitting provides roughly the same benefit as taking 3 medications together.
The agent is the first oral therapy for this indication.

Investigational CAR T-cell therapy is currently being evaluated in an ongoing dose escalation phase 1 trial to analyze its safety and tolerability in treating non-Hodgkin lymphoma.
Elaine Jaffe, MD, National Institutes of Health (NIH) distinguished investigator at the National Cancer Institute at NIH, discusses where she sees the classification of lymphoma progressing in the coming years.
Among Black and Latina women with heart failure, negative health outcomes are magnified due to significant health disparities and inequities in the management of the chronic condition.
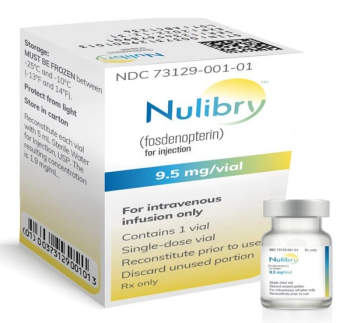
Fosdenopterin (Nulibry) is indicated to reduce the risk of mortality in patients with molybdenum cofactor deficiency Type A.
Sonia Oskouei, PharmD, vice president of biosimilars at Cardinal Health, discusses what biosimilars in the pipeline are expected to launch this year.

Analyses are shared at the American Association for Cancer Research Annual Meeting 2022 in New Orleans, Louisiana.

Sonia Oskouei, PharmD, vice president of biosimilars at Cardinal Health, on the results of Cardinal Health’s assessment of the current biosimilars market published in the 2022 Biosimilars Report.

Although the ISMP created the guidelines for hospitals, they are applicable in other health care settings.

Additionally, the safety profile in DESTINY-Lung01 was consistent with previous clinical trials with no new safety concerns identified.
Asciminib offers a new option for patients harboring the T315I mutation.
Session highlights novel agents that have recently entered, or will imminently enter, into phase 1 clinical trials.

Medicaid expansion implementation was associated with a decrease in the number of uninsured patients from 6.7% pre-expansion to 3.6% post-expansion.
MIS-C is a rare complication of SARS-CoV-2 infection, which is characterized by symptoms such as fever, multiorgan involvement, and documented SARS-CoV-2 exposure.
Elaine Jaffe, MD, National Institutes of Health (NIH) distinguished investigator at the National Cancer Institute at NIH, discusses points of note in the evolution of lymphoma classification during her clinical and investigational research on the subject.

Heart-related complications in children with COVID-19 are uncommon, but case reports have noted cardiogenic shock, myocarditis, pericarditis, and arrhythmias.

Sonia Oskouei, PharmD, vice president of biosimilars at Cardinal Health, on the results of Cardinal Health’s assessment of the current biosimilars market published in the 2022 Biosimilars Report.
David DeRemer, PharmD, BCOP, FCCP, FHOPA, a past-president on the board of Hematology/Oncology Pharmacy Association (HOPA), discusses why a greater focus on colorectal cancer screening was important to highlight at the HOPA 2022 annual conference.

Bevacizumab-maly (Almysys; mAbxience) is the third biosimilar of bevacizumab (Avastin) approved in the United States.

David Pope, PharmD, CDE, chief innovation officer at OmniSYS, discusses some of the technology trends that occurred in the pharmacy space during the pandemic.
An earlier clinical trial found a higher number of acute myocardial infarction events in those administered HepB-CpG vaccine versus those administered the HepB-alum vaccine.

Rita Roy, MD, CEO of the National Spine Health Foundation, discusses some alternative treatment plans for chronic back pain to help address the opioid epidemic.

Dhivy is a levodopa/carbidopa combination formulation approved by the FDA in November 2021 for the treatment of Parkinson disease or manifestations due to viral or environmental exposures.

New therapies and combination regimens may allow a more tailored approach.

David DeRemer, PharmD, BCOP, FCCP, FHOPA, a past-president on the board of Hematology/Oncology Pharmacy Association (HOPA), discusses key takeaways from the HOPA 2022 annual conference.
CDI is the leading cause of diarrhea in hospitalized patients.
PrEP use has been associated with lowering HIV infections worldwide, however use of the treatment alone is not sufficient to prevent HIV from spreading.