
Service outcomes will be optimized by considering a constellation of factors besides the service itself, such as social support and patient self-efficacy.

Service outcomes will be optimized by considering a constellation of factors besides the service itself, such as social support and patient self-efficacy.
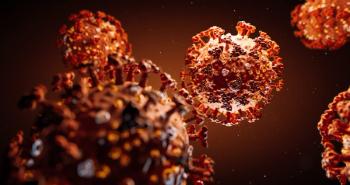
In the United States, the CDC has confirmed 938 cases and 29 deaths, as well as 771 patients under investigation.

As vaping continues to grow in popularity, it becomes increasingly critical to correct misconceptions about its safety and long-term effects.

The FDA is taking action against companies that claim to treat or prevent the virus.
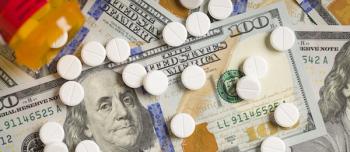
Health care spending increased by 4.4% in 2018, slightly more than the 4.2% increase in 2017, and the third consecutive year with an increase greater than 4%.

Rotovirus infection is a hypothesized risk factor for type 1 diabetes, according to the authors.

The act aims to mitigate common triggers of drug shortages by improving transparency throughout the drug supply chain process, and strengthening FDA interagency efforts to prevent shortages.

Pharmacy managers invariably face employees suffering from various personal issues that require special attention and/or counseling, and at some point will be confronted with the issue of an impaired employee.
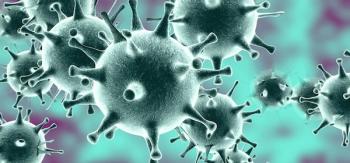
The greatest risk is still to people who have been in close contact with COVID-19 patients, according to the CDC.

If severe, toxoplasmosis can cause damage to the brain, eyes, or other organs.

A study published in Neuron suggests that targeting astrocyte calcium signaling could decrease the behavioral effects of amphetamine.

The product will be immediately launched with limited commercial qualities, and manufacturers expect that there will be a steady supply by the fourth quarter of 2020.

The new drug is a nonopioid therapeutic option for patients experiencing moderate to severe pain.

Fears surrounding the COVID-19 outbreak have had major effects even in unaffected areas, including face mask shortages, a falling stock market, and many unsubstantiated rumors.

Pharmacists are the primary drivers of their destiny, according to a new report, and they will continue to drive change in the industry by demonstrating their value, securing compensation, and advocating for expanded roles.
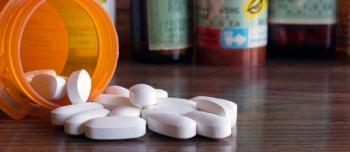
Over 90% of hospice and palliative care patients are prescribed opioid medications for pain management, and many individuals receive home care.
Results from a new retrospective analysis have shown that patients prescribed tiotropium bromide (Spiriva Respimat) inhalation spray 1.25 mcg experienced fewer asthma-related exacerbations when the treatment was added to a combination inhaled corticosteroid and long-acting beta2 agonist (ICS + LABA), compared with patients who received an increased dose of ICS + LABA.

Although the number of US cases is still low, experts are raising concerns that the US reliance on China for pharmaceutical ingredients could result in drug shortages as Chinese officials battle the outbreak.

Megan Maroney, PharmD, BCPP, clinical associate professor, Ernest Mario School of Pharmacy at Rutgers, the State University of New Jersey; clinical psychiatric pharmacist, Monmouth Medical Center, Long Branch, NJ, discusses how to navigate data on medications with higher risks of suicidality and depression.

This week’s tip examines a comprehensive set of pharmacist-based interventions in acute care settings.

Community pharmacy antibiotic dispensing data were obtained from the New Zealand Pharmaceutical Collection database for children whose parents consented to external data linkage.
Several immunization recommendations were updated for pediatric patients, including haemophilus influenza type b (Hib) vaccination, and Tdap vaccinations.
Current recommendation of beta blockers in patient with HF include bisoprolol, carvediol, or metoprolol succinate. Metoprolol tartrate may provide better rate control with twice daily dosing.

Each year, infections related to antibiotic-resistant superbugs kill 700,000 people worldwide, according to researchers at the Italian Society of Anti-Infective Therapy (SITA).

Vizient is taking a strategic approach to lowering drug costs and shortages with the recent creation of an Essential Medicines list.

Both semaglutide and dulaglutide are dosed once weekly for treatment of type 2 diabetes.

Tedros Adhanom, PhD, director general of WHO, concluded his statements in the WHO press conference by reiterating that while 2019-nCoV is of global concern, it should not lead to widespread panic.

Users will be able to export reports and collect metrics with the new interactive platform, as well as readily access the information on mobile platforms.

Megan Maroney, PharmD, BCPP, clinical associate professor, Ernest Mario School of Pharmacy at Rutgers, the State University of New Jersey; clinical psychiatric pharmacist, Monmouth Medical Center, Long Branch, New Jersey, discusses several risk factors for patients on medications with higher risks of suicidality and depression.