
Two lawsuits against the FDA have been resolved in a conclusion favorable to Endo International, which argued that vasporessin should be excluded from the agency’s compounding list.

Two lawsuits against the FDA have been resolved in a conclusion favorable to Endo International, which argued that vasporessin should be excluded from the agency’s compounding list.

Many critics have accused Oliver of oversimplifying a complex issue.

Along with her B.S. in pharmacy from UNC-Chapel Hill, author Amy Greeson’s education has included some much more unique experiences. She’s been to Belize to study native therapies, studied in the Amazon with a local medicine man and woman, and learned from traditional healers in Mexico.

The FDA has given the green light to rituximab (Rituxan) injection for the treatment of granulomatosis with polyangiitus (GPA) and microscopic polyangiitis (MPA) in children age 2 and older in combination with glucocorticoids. The application was approved under priority review and with orphan designation.

Ahead of World Heart Day, Amgen has released a study demonstrating that patient education is still necessary to combat cardiovascular disease.
The FDA approved Jynneos (Bavarian Nordic) as the first live, nonreplicating vaccine to prevent smallpox and monkeypox.

On September 25, 2019, millions of pharmacists worldwide will celebrate World Pharmacists Day, this year themed “safe and effective medicines for all.”

After some lots of ranitidine were found to contain small amounts of N-nitrosodimethylamine (NDMA), Novartis's Sandoz unit has announced that they are halting distribution of ranitidine (Zantac) products until the contamination concerns are resolved.

Walgreens is set to be the first retailer in the US to test on-demand drone delivery services by partnering with Wing Aviation LLC, a member of the Alphabet family of companies.

The FDA has granted approval of Rybelsus (semaglutide) oral tablets 7 mg or 14 mg to Novo Nordisk. The drug is the first oral glucagon-like peptide (GLP-1) receptor protein treatment for type 2 diabetes.
The new report found that widely used prescription drugs increased by an average of 4.2% in 2017, double the general inflation rate of 2.1%.

The FDA has approved Erleada (apalutamide) for the treatment of metastatic castration-sensitive prostate cancer (mCSPC), based on the results from the Phase 3 TITAN study.

Hematology and oncology pharmacists discussed patient outreach at a panel during the Hematology/Oncology Pharmacy Association’s (HOPA) annual conference in April.

Researchers have found that methylnaltrexone, a peripherally acting µ-opioid receptor antagonist, may offer an effective solution to relieving patients' opioid-induced constipation with minimal adverse effects.

As an increasing number of youth are using e-cigarette products, the FDA has announced a federal plan intended to clear the market of unauthorized, nontobacco-flavored e-cigarette products.

London Mayor Sadiq Khan has called for broader availability of pre-exposure prophylaxis drugs in his welcoming speech at the Fast-Track Cities 2019 conference, where leaders from 300 cities around the world will discuss municipal responses to HIV, tuberculosis, and viral hepatitis.

The FDA has issued a warrning letter to JUUL Labs Inc. regarding marketing and advertising practices.
The product to treat life-threatening allergies arrives amid the ongoing supply shortage.

Caring for patients in a rural community is vastly different than in a city, and students at 2 universities got a chance to see the differences firsthand earlier this year.

Manufacturers are releasing their own products to meet demand amid the EpiPen shortage.

Johns Hopkins Medical has announced a new Center for Psychedelic and Consciousness Research, funded with $17 million from private donors.
Positive Phase III study results have shown that preventive flu treatment with baloxavir marboxil after exposure to an infected household member reduced the risk of contraction by 86% versus a placebo, according to Roche.
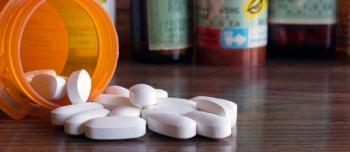
Researchers at Weill Cornell Medicine have found that states requiring prescribers and dispensers to register with and use prescription drug monitoring programs saw significantly fewer opioid prescriptions and reduced opioid-related hospital use, when compared to states with weak drug monitoring program mandates.

A federal court has ordered 2 Tennessee-based companies with the same owner to stop disstributing drugs, dietary supplements, and devices until they are found to be in compliance with the Federal Food, Drug, and Cosmetic (FD&C) Act and other requirements.

As the industry moves toward a more patient-centric model, pharmacy schools like Touro are implementing programs to ensure their students are prepared for every aspect of their career—including physical exams.

A randomized clinical trial found that among patients with psychotic depression in remission, continuing sertraline plus olanzapine reduced the risk of relapse over 36 weeks when compared with sertraline plus a placebo.

The multicultural population is increasing and thus creating a vital new market for pharmacies looking to grow and expand their services, said Mariko Carpenter, VP of Strategic Community Alliances at Nielsen, in a session at the 2019 NACDS Total Store Expo in Boston.

H-E-B, a supermarket chain based in San Antonio, Texas, received the 2019 PEP Award at a reception Friday evening in Boston, as part of the NACDS Total Store Expo.

John King, CEO, and David Pope, Chief Innovation Officer, of OmniSYS, discuss how they're tackling reimbursement issues for pharmacies.

Former Secretary of State John Kerry spoke about the need for bipartisan and international support during a business session at the 2019 NACDS Total Store Expo in Boston.