Senator Scott Wiener, discusses the California Senate Bill 519 he co-authored, which is the first and most expansive psychedelic decriminalization bill to go through a state legislature.

Senator Scott Wiener, discusses the California Senate Bill 519 he co-authored, which is the first and most expansive psychedelic decriminalization bill to go through a state legislature.
In an interview with Pharmacy Times®, Maria Whitman, Global Head of the Pharmaceutical and Biotech Practice at ZS, discusses the FDA’s Project Optimus, the FDA’s new guidance addressing dose optimization issues in oncology clinical trials, and how it has impacted drug manufacturers.

In a Pharmacy Times® interview, Jessica Hui, a researcher and pediatric allergist at National Jewish Health, discussed how increased hand hygiene may be harming the hands of healthcare workers.
Donna Bohannon, RPh, MS, principal scientist at US Pharmacopeia (USP), and Bhumi Khambholja, PharmD, MSHI, project manager of Wisconsin Health Literacy, discuss how to adopt USP standards for labeling to increase health equity.

Kelan Thomas, PharmD, MS, associate professor of clinical sciences at Touro University California College of Pharmacy, discusses potential adverse effects and drug-drug interactions with psilocybin, MDMA, and ketamine treatment.

Lauren C. Pinter-Brown, MD, FACP, of UCI Health and Chao Family Comprehensive Cancer Center, explains what the role of the pharmacist is during the treatment of patients with advanced cutaneous T cell lymphoma.

Lauren C. Pinter-Brown, MD, FACP, of UCI Health and Chao Family Comprehensive Cancer Center, addresses the areas that may need further investigation to progress advanced cutaneous T cell lymphoma treatments in the future.

Bhavesh Shah, RPh, BCOP; and Thomasina Morris, RPh, MHA, BCOP, discuss the mechanism of action and efficacy for the 3 PARP inhibitors used to treat patients with ovarian cancer: olaparib, rucaparib, and niraparib.

Bhavesh Shah, RPh, BCOP, provides a brief history of PARP inhibitors in the cancer space, as well as his own introduction to PARP inhibitors.

Hans Eriksson, MD, PhD, the chief clinical development officer at HMNC Brain Health, on the development of personalized treatments for neuropsychiatric disorders using precision-medicine.
Lauren C. Pinter-Brown, MD, FACP, of UCI Health and Chao Family Comprehensive Cancer Center, explains what research is underway in the advanced cutaneous T cell lymphoma field that she is currently keeping an eye on.
Shawn Singh, CEO of VistaGen, discusses how to improve and more effectively treat mental health disorders for returning veterans, who currently account for 20% of all suicides in the United States.

Laura Lee Hall, PhD, president of the Center for Sustainable Health Care Quality and Equity (SHC), discusses the SHC’s recently released Flu DRIVE Toolkit, which was designed to help overburdened health systems improve flu vaccination rates among vulnerable populations.

Lauren C. Pinter-Brown, MD, FACP, of UCI Health and Chao Family Comprehensive Cancer Center, describes some of the challenges providers face during the treatment of patients with advanced cutaneous T cell lymphoma and how to address them.
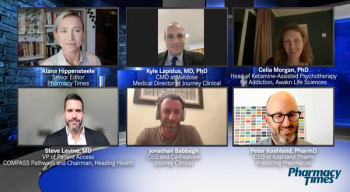
A panel of experts in the field of ketamine therapy discuss the role of the pharmacist in this field and the future of ketamine and assisted therapies based on current medical research.

Lauren C. Pinter-Brown, MD, FACP, of UCI Health and Chao Family Comprehensive Cancer Center, addresses how the treatment of advanced cutaneous T cell lymphoma has changed in recent years and whether there have been any advancements of note.

Andrea Silber, MD, said the COVID-19 pandemic has exacerbated disparities that already existed within oncology care, and she said the effects of these disparities on patients are very real and direct.

Lauren C. Pinter-Brown, MD, FACP, of UCI Health and Chao Family Comprehensive Cancer Center, provides an overview of key trials that have been conducted in the advanced cutaneous T cell lymphoma space in recent years.

In part 2 of their discussion on COVID-19 vaccine mandates, experts Ned Milenkovich, PharmD, and Ron Lanton III, Esq., discussed how provider status could impact potential legal issues around COVID-19 vaccine mandates.
Immune globulin subcutaneous (human), 20% solution (Cuvitru) is approved for primary immunodeficiency in patients 2 years of age and older.

Thomas Menighan, co-founder of PursueCare and the CEO Emeritus of the American Pharmacists Association, discusses a pharmacists role in National Recovery Month.
Lauren C. Pinter-Brown, MD, FACP, of UCI Health and Chao Family Comprehensive Cancer Center, explained whether the current advanced cutaneous T cell lymphoma (CTCL) treatment strategies available are applicable for all patients with advanced CTCL and how patients are selected for treatment.

With the recent announcement of federal COVID-19 vaccine mandates, in addition to private businesses potentially requiring vaccines for employees, pharmacies and other health care facilities could have questions about legal issues surrounding these mandates.

Lauren C. Pinter-Brown, MD, FACP, of UCI Health and Chao Family Comprehensive Cancer Center, described what advanced cutaneous T cell lymphoma is in terms of classification and what current treatment approaches are available.

In The Legal Prescription series with Ned Milenkovich, we discuss a set of important topics in the pharmacy landscape.
Sophia Humphreys, PharmD, MHA, said data are continuing to evolve around the safety and recommendations of COVID-19 booster doses, especially for the vaccine from Pfizer and BioNTech.
Gemtesa (vibegron; Urovant Sciences) tablets are approved to treat overactive bladder with symptoms of urge urinary incontinence, urgency, and urinary frequency.

Pharmacy Times spoke with Dr. Bradley Monk, investigator for KEYNOTE-826, #LBA2, which was presented at the ESMO Congress 2021, about the study and its importance to the world of cervical cancer.
Shereen Stutz and Mark Alwardt, of McKesson, said the rise in oral oncolytics during the COVID-19 pandemic allowed pharmacists to be more innovative and to become an even more integrated part of the oncology care team.

September is National Recovery Month, so Pharmacy Times interviewed Rich Dion, PharmD, of Wolters Kluwer, Health, on the critical role of the hospital pharmacist in ongoing efforts to fight the opioid epidemic.