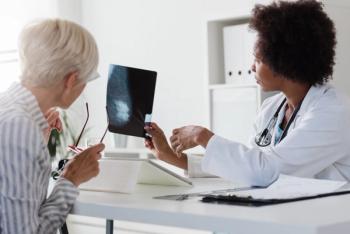
New findings on nodal radiation for breast cancer patients highlight the importance of lymph node involvement and tumor size in treatment decisions.

New findings on nodal radiation for breast cancer patients highlight the importance of lymph node involvement and tumor size in treatment decisions.

Discover how GLP-1 receptor agonists may benefit breast cancer patients by promoting weight loss and improving long-term health outcomes.

Explore the latest insights on breast cancer treatment, focusing on radiation, endocrine therapy, and the role of pharmacists in patient care.
This abstract will be presented at the Oncology Pharmacists Connect (OPC) meeting in Austin, Texas, from June 19 to 20, 2025.
This abstract will be presented at the Oncology Pharmacists Connect (OPC) meeting in Austin, Texas, from June 19 to 20, 2025.
Vincent Chung, MD, discusses key findings from the NAPOLI-3 trial and offers guidance for pharmacists on managing toxicities, adjusting dosing, and supporting patients receiving NALIRIFOX for metastatic pancreatic ductal adenocarcinoma.
Hope Rugo, MD, discusses a multi-institutional real-world analysis showing that rechallenging trastuzumab deruxtecan after grade 1 interstitial lung disease (ILD) is generally safe and effective.

Patients in a Phase 3 clinical trial who received sotorasib and panitumumab lived longer, suggesting the combination therapy could become the new standard of care.

Delayed pegaspargase reduces hepatotoxicity without affecting outcomes.

Wafa Samara, PharmD, vice president and chief pharmacy officer at City of Hope, highlights the vital role of oncology pharmacists at City of Hope in improving patient outcomes, conducting and leading research, and working as integral members of the multidisciplinary care team.
Edward Kim, MD, MBA, discusses critical points of patient leakage in precision medicine, emphasizing the role of multidisciplinary teams in streamlining care, managing medications, and improving patient outcomes.
Edward Kim, MD, MBA, highlights the challenges of patient disengagement, data fragmentation, and provider education in precision medicine, emphasizing the need for personalized approaches to enhance patient outcomes, particularly in oncology.
Leukemia biology may predict patterns of blinatumomab failure after initial response.

Jose Tinajero discusses how certain mutations can be indicators of blinatumomab treatment failure in patients with B-cell acute lymphoblastic leukemia.

New research by City of Hope suggests the biotherapeutic product, CBM588, adjusts people’s microbiome, possibly leading to enhanced effectiveness of FDA-approved cancer immunotherapies.

Research focused on potentially reducing hypoglycemic episodes, why more men might have diabetes than women, and targeting glucose control.

Trial results published today in Nature Medicine and a phase 1b trial using the same CAR T cell therapy has opened.

Aimee Keegan, PharmD, BCOP, discusses key unmet needs that the NATALEE trial addresses for patients with HR+/HER2- early breast cancer.

A clinical pharmacist details how treatment algorithms differ between early-stage and metastatic breast cancer.

Expert perspectives on differentiating between the three CDK4/6 inhibitors available for the treatment of metastatic breast cancer are discussed, highlighting factors that inform treatment selection.

Aimee Keegan, PharmD, BCOP, discusses how her institution optimizes patient adherence to CDK4/6 inhibitors, highlighting resources including their in-house specialty pharmacy.

Focusing on metastatic breast cancer, Aimee Keegan, PharmD, BCOP, provides clinical insights on ensuring optimal adherence to CDK4/6 inhibitors.

The vice president and chief pharmacy officer at City of Hope explains how she serves as a mentor for women within health care, the importance of networking, and staying educated.

The vice president and chief pharmacy officer of City of Hope discusses obstacles women face in health care and how diversity, equity, and inclusion can be promoted within the space.
“I see clinical pharmacy specialists (CPS) to be more like the frontline folks that would pretty much work very closely with the providers,” according to a CPS at City of Hope.

The model implemented at City of Hope Chicago has been shown to improve provider workloads and enrich pharmacists’ work.

Erica Marchese, PharmD, MHA, BCPS, BCOP, BCSCP, discussed the revision of pharmacy and the contributing roles within the pharmacy team.

Kimlin Tam Ashing, PhD, discusses her research assessing effective interventions in ethnic minority communities to increase colorectal cancer screenings.

Branding Move and Leadership Additions Part of Effort to Deliver “One City of Hope” Experience to Patients Across National Clinical Network.

CTCA leverages new CMS Conditions of Participation notification requirements as opportunity for innovation.