
PQA Health Equity Technical Expert Panel recommendations inform measure testing, use in quality programs, and risk adjustment models

PQA Health Equity Technical Expert Panel recommendations inform measure testing, use in quality programs, and risk adjustment models

Dicyclomine Hydrochloride Capsules, USP are an antispasmodic and anticholinergic (antimuscarinic) agent indicated for the treatment of functional bowel/irritable bowel syndrome.

But Congress must ignore big insurance and the PBM lobby’s last-ditch effort to scuttle reforms and pass the continuing resolution

Erlotinib Tablets are available in 25 mg, 100 mg, and 150 mg strengths, packaged in 30-count bottles.

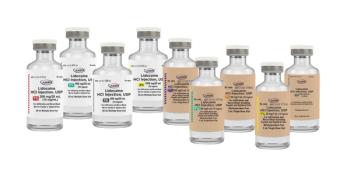
Featuring 1% and 2% concentrations, this versatile product is available in various vial and pack sizes to meet diverse healthcare needs.

It is the only immuno-oncology drug approved by various regulatory authorities around the world such as the USFDA, DCGI, EMA, MHRA, NMPA and others for the treatment of adults with recurrent or metastatic nasopharyngeal carcinoma (RM-NPC).

Key Appointment Focused on Driving Innovation in Pharmacy Inventory Management

Given the unfair aspects of CVS’s arbitration clause, delegating to an arbitrator to decide whether the case has to be arbitrated is “unconscionable,” says federal judge

Making America Healthy Again should start with PBM reform














Camber Pharmaceuticals is proud to expand its product line with Quetiapine Tablets, USP, offering patients a more affordable, high-quality treatment option.

Camber is the first and only generic option for Gastrografin®.

This marks India’s first trial for a novel autologous BCMA directed CAR-T cell therapy in patients with relapsed/refractory multiple myeloma.

CMS’ failure to require timely, sufficient pharmacy reimbursement will lead to closures not seen since the start of Part D and patients will suffer as a result.


With a scalable, flexible suite of services and an experienced leadership team, InspiroGene enables manufacturers, payers, and providers to navigate the complex CGT commercialization landscape to ensure patients can access the life-changing treatments they need.