
With a coordinated, interprofessional team supporting them, patients are empowered with information at the point-of-care to help manage medication therapy problems as they arise.

With a coordinated, interprofessional team supporting them, patients are empowered with information at the point-of-care to help manage medication therapy problems as they arise.

Moderna is completing regulatory submissions for mRNA-1273.214 in preparation for the fall booster season, based on clinical data showing that the booster candidate has a significantly higher neutralizing antibody response against all tested variants including Omicron BA.4/5.

From the first approved CAR T therapy for multiple myeloma, to the recently-approved ciltacabtagene autoleucel, significant steps have been taken.

The FDA has approved fam-trastuzumab deruxtecan-nxki (Enhertu) for previously treated HER2-positive breast cancer and gastric or gastroesophageal junction adenocarcinoma.

The FDA's current way of inspecting facilities for quality control is badly outdated and is politically unworkable, unreliable, and unsafe.

A booster dose of both Omicron-adapted vaccine candidates elicited a substantially higher immune response against Omicron BA.1.

Community pharmacists are vulnerable to security threats, resulting in bad outcomes for both patients and pharmacies. Here’s how to evaluate risk and find the right technology partner to help protect and defend you from cybercriminals.

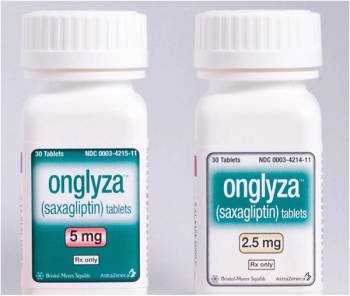
Saxagliptin (Onglyza) is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.

ThermaCare is intended to provide relief from menstrual pain.
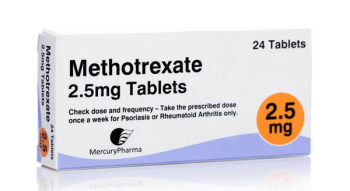
Methotrexate is an immunosuppressive agent used to treat cancer, dermatology-, and rheumatology-related issues.

Vibhuti Arya Amirfar, PharmD, MPH, discusses building individual and systems resilience to dismantle structural racism in the pharmacy field.
Though most primary care physicians indicate a willingness to accept expanded recommendations from the Advisory Committee on Immunization Practice suggesting administration of recombinant zoster vaccine for adult patients who are immunocompromised, cost concerns and knowledge gaps limit vaccine delivery.

Experts discuss tools and trainings that health care institutions have established during the pandemic to support hematology-oncology pharmacists who are struggling with wellness and mental health challenges.
Screening participation increased over time, but the increase was smallest among individuals aged 50 to 54.

Study results show that there is a link between leaving the television on, night lights, and smart phones and higher disease rates.

An intelligence-based system could help improve the timeliness of referrals to palliative care and hospice services in a community oncology environment.

The American Diabetes Association guidelines have self-care and lifestyle recommendations for patients and health care professionals.

On this week's episode, Joanna speaks with Carlene Link, PharmD, a clinical pharmacist and integrative life coach, about burnout, managing mental health, and the state of pharmacy.
Hepatitis-associated emergency department visits and hospitalizations, liver transplantations, and adenovirus stool testing results in pediatric patients were not found to increase during the COVID-19 pandemic.
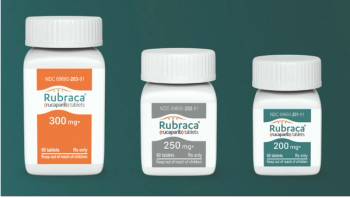
Rucaparib (Rubraca) is a poly (ADP-ribose) polymerase (PARP) inhibitor used for ovarian cancer, fallopian tube cancer, primary peritoneal cancer, and castration-resistant prostate cancer.

Instant Sleep is intended to aid with falling asleep faster and staying asleep longer.

Learning the Pharmacists' Patient Care Process steps and being mindful or internalizing those can help set best practices for pharmacy team members.
The non-estrogen pill was first approved by the FDA in 1973.

Joe Duarte, co-founder and president of ContinuumMD, discusses a program model that allows pharmacies to provide virtual and in-person primary care options for rural communities with health care access challenges.

Patients with refractory chronic migraine experience short- and medium-term pain relief and minimal adverse drug effects from lidocaine infusions.
Subcutaneous immunotherapy-induced antibodies may serve as a potential allergen-targeted biologics candidate for the treatment of allergic asthma.
Christopher Fine, MD, FACC, a cardiologist at National Jewish Health, discusses how pharmacists can support patients with cancer who have a much higher risk of heart disease.

Health Mart also presented the first ever Innovation Award at McKesson ideaShare 2022.

Lifestyle modifications are the cornerstone of treatment.