
Top news of the week from Specialty Pharmacy Times.

The approval of fedratinib (Inrebic, Celgene) provides another treatment option for patients with myelofibrosis, a rare bone marrow disorder.

Officials with the FDA have granted approval to entrectinib (Rozlytrek, Genentech) for 2 different cancer indications.

The dug will be commercially available to healthcare professionals and appropriate patients in the U.S. in the fourth quarter of 2019.

Top news of the day from across the health care landscape.

HIV-related immunosuppression may contribute to an elevated risk of cancer-related morbidity in elderly patients with the disease.

If approved, acalabrutinib (Calquence, AstraZeneca) will offer a chemotherapy-free treatment option for adults with chronic lymphocytic leukemia.

An analysis of a quality improvement project at an oncology specialty pharmacy showed that pharmacist interventions can reduce financial toxicity for patients.

Top news of the day from across the health care landscape.

High body mass index is associated with an increased risk of chronic diseases such as type 2 diabetes, cancer, musculoskeletal, and respiratory conditions.
Officials with the FDA have approved Alembic Pharmaceuticals’ Fenofibrate Tablets USP, 48 mg and 145 mg as an adjunctive therapy to diet to reduce elevated low-density lipoprotein cholesterol (LDL-C), total cholesterol (Total-C), Triglycerides and apolipoprotein B (Apo B).

Artificial intelligence able to recognize patterns in gene sequences and molecular data from breast cancer, which oncologists are now evaluating in clinical trials.
A phase 3 trial evaluated dupilumab (Dupixent, Regeneron and Sanofi) in children from 6 to 11 years of age with severe atopic dermatitis.

Alembic Pharmaceuticals has received FDA approval for Dorzolamide Hydrochloride Ophthalmic Solution USP, 2%, therapeutically equivalent to Trusopt Ophthalmic Solution, 2% (Merck Sharp & Dohme Corp.).

Top news of the day from across the health care landscape.

Genomind is expanding its mental health genetic testing services with the addition of Genomind Professional PGx Express, which provides a more focused report and set of services to enhance the preexisting mental health genetic test.
The number of first-degree relatives affected by blood cancer and age of diagnosis may contribute to the likelihood of a family member’s risk of being diagnosed with the same disease.

Investigators believe that urine sampling can be used to diagnose and treat bladder cancer.

Top news of the day from across the health care landscape.
In an ideal world, how would you hypothesize that technology might be able to solve all of your problems?

What is causing the suspicious MRI results?

An FDA committee has recommended approval of Gilead’s Descovy to reduce the risk of sexually acquired HIV infection in those at high risk of acquiring the virus.

An FDA committee voted 16 to 2 to recommended emitricitabine 200 mg and tenofovir alafenamide 25 mg tablets (Descovy, Gilead) for a HIV pre-exposure prophylaxis indication.
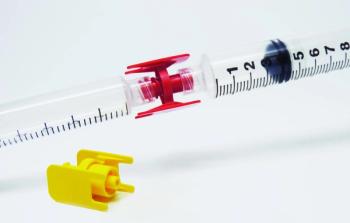
These new Guarded Luer Connectors are used to join two luer lock syringes or other luer lock devices.

As new pharmacists receive their exam results and enter the workforce, many are facing questions about their school experiences, career plans, further education plans, and the rising debt students face.

Galcanezumab-gnlm (Emgality, Eli Lilly) may be an effective option for patients who failed previous migraine preventive treatments.

Top news of the week from Specialty Pharmacy Times.

Top news of the day from across the health care landscape.

Physicians’ Education Resource® (PER®), a worldwide leading resource for continuing medical education (CME), named public health expert and former commissioner of the Food and Drug Administration (FDA) Scott Gottlieb, M.D., as the keynote speaker for the 37th Annual Chemotherapy Foundation Symposium Innovative Cancer Therapy for Tomorrow.®

The phase 3 IMvigor130 study is the first to assess a cancer immunotherapy combination in previously untreated advanced bladder cancer.