
Daniel Gagnon, VP of Global UPS Healthcare, discusses vaccine equity panel discussion at the UPS Impact Summit in Atlanta, which was held at the National Center for Civil and Human Rights.

Daniel Gagnon, VP of Global UPS Healthcare, discusses vaccine equity panel discussion at the UPS Impact Summit in Atlanta, which was held at the National Center for Civil and Human Rights.

A variety of cannabidiol (CBD) products incorrectly listed their CBD and tetrahydrocannabinol content, in addition to making therapeutic and cosmetic claims without FDA approval.

How would you handle these patient questions?

When pharmacists are involved in the care process, they can address common barriers to medication use, such as polypharmacy, ineffective medication, high costs, health and lifestyle considerations, and social needs.

This month's featured products include prenatal supplements, an intensive hydrating serum, and more.

COVID-19 vaccination averted infection in 10% of NHS health care workers and reduced absence from work by nearly 70%.

When we talk about access, that is a pharmacist.

Fewer than half of eligible veterans enrolled in the Veterans Health Administration have received their COVID-19 booster.
Results of analysis also show they have a much higher incidence of dying in the hospital than those without the virus.
The numbers dropped sharply in March 2020 from before the pandemic among men who have sex with men and individuals who inject drugs.

This month, we tackled how to find and utilize a mentor both as a pharmacy student and after graduation.

Tapinarof (Vtama) cream, 1% is an aryl hydrocarbon receptor agonist indicated for the topical treatment of plaque psoriasis in adults.
AfterBurn gel offers relief for sunburn, scalds, and other types of burns.
Combining tramadol and celecoxib into a co-crystal of celecoxib-tramadol provides peripherally and centrally mediated analgesia.

Health Mart University also offers "basic technician training programs," to allow pharmacies to bring those new technicians on board, help them meet the PTCB examination standards, and really get that new technician ready to go.

From a patient perspective, the lack of awareness of biosimilars is the biggest barrier to adoption.

This month's featured products include viloxazine extended-releast capsules, lumateperone, and more.
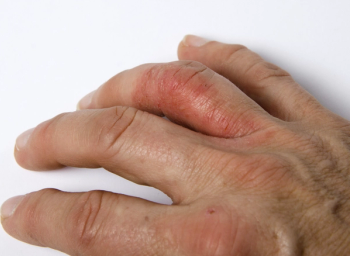
People living with psoriatic arthritis deserve to be informed about ways to approach disease management holistically and with a personalized approach.

This is important to establish when deciding on a pharmacy focus or making a career change.

Along with expanding responsibilities, pharmacies must have the infrastructure and pharmacy staff must have sufficient support.

Unvaccinated first responders were more likely to doubt the safety and efficacy of COVID-19 vaccines than vaccinated counterparts, reporting low trust in the government.
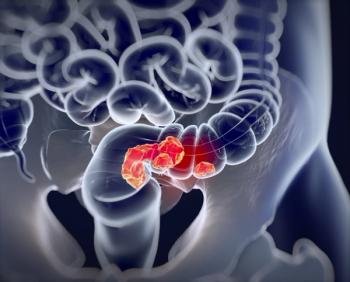
Delayed diagnosis in younger individuals and presentation of advanced disease highlights need for awareness.

Participants took about 50 times higher than the recommended daily allowance every day for a month, investigators say

Once the revisions to <797> become approved, there will be a minimum grace period of 6 months for pharmacies to comply.
Kevin Straughn, PharmD, clinical pharmacist at Duke Regional Hospital, said once the 2 compounding chapters, <797> and <795> become enforceable, there will be a 6-month grace period before USP <800> becomes enforceable.

Piperacillin and tazobactam (Zosyn) is indicated for treatment of intra-abdominal infections, skin and skin structure infections, female pelvic infections, community-acquired pneumonia, and nosocomial pneumonia.
Alocane provides fast pain relief and helps the healing process from minor burns.

Health Mart was able to deliver the targeted education they needed in a virtual manner to allow them to start in the uniting and to keep their immunization credentials.

We need to be able to build on that momentum, and at the same time, we need sustainable reimbursement models.

Health Mart University started as a platform to be franchise standards.