Sarcopenia, a progressive skeletal order that involves the accelerated loss of muscle mass and function, is associated with adverse outcomes such as functional decline, frailty, and mortality.

Sarcopenia, a progressive skeletal order that involves the accelerated loss of muscle mass and function, is associated with adverse outcomes such as functional decline, frailty, and mortality.
The novel formulation of trametinib has the potential to change the treatment paradigm of human papillomavirus (HPV) and prevent infections before they progress to deadly cancers.

The cancers linked with per- and polyfluoroalkyl substance (PFAS) exposure included nonastrocytoma gliomas, acute myeloid leukemia, and Wilms tumors.

Michael McGuire, PharmD, highlights the importance of early intervention in hospital settings, leveraging manufacturer programs, and navigating complex dosing regimens.

The treatment is currently undergoing evaluation in an investigational preclinical program and is projected to be in human trials in 2026.
A team of clinical and academic experts from the Association of Anaesthetists produced a consensus statement outlining the key components to minimizing the impact of harmful alcohol intake in the surgical population.

A pilot program integrated oncology pharmacists into prior authorization workflows to streamline and expedite medication approvals.

Subcutaneous infusion reduces treatment times for patients and eases operational burdens for providers.

Policymakers and health care providers call for reforms to improve transparency, pricing, and patient access.

Specialty pharmacists are critical for enhanced management of clinical and logistical aspects of treatment.

The treatment's expanded indication for chronic kidney disease (CKD) is expected to be available by April 2025.

A brief period of overeating ultra-processed, high-calorie snacks could lead to liver fat accumulation and temporary disruption of brain insulin action.
Efficacy and tolerability of topical treatments can vary by region, particularly sensitive areas such as the face and neck.

Melatonin supplementation could improve oxidative DNA damage repair capacity among night shift workers.
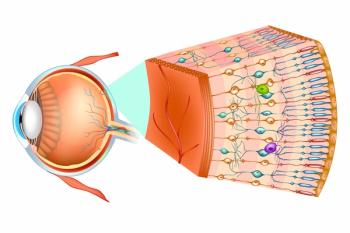
With the new designation, OpCT-001 can receive expedited investigations into its potential to improve vision function in patients with primary photoreceptor diseases.
At 1- and 2-year follow-up points, ruxolitinib led to considerable improvements in survival in patients with myelofibrosis who develop graft-versus-host disease (GVHD).
The approval is based on results from 2 phase 3 clinical trials that showed revakinagene taroretcel’s efficacy in macular telangiectasia type 2 (MacTel).

The move is expected to expand clozapine access for patients with schizophrenia.

According to a news release, this marks the first significant innovation in epinephrine delivery for this patient population is more than 35 years.

Elevidys offers significantly higher dystrophin production than existing Duchenne muscular dystrophy treatments but requires intensive monitoring for adverse events.

Unlike other professionals such as nurses and physicians, pharmacists remain tethered to a fragmented state-by-state licensure model that creates unnecessary barriers to care.

Adalimumab biosimilars differ from adalimumab in approved indications, concentration and dosage, and formulation.
Camizestrant is an investigational oral selective estrogen receptor degrader.

Dogs share a number of cancers with humans, including melanoma, non-Hodgkin lymphoma, leukemia, and osteosarcoma.

The authors’ findings show a greater decline in those treated with chemotherapy vs endocrine therapy.
Following allogeneic hematopoietic stem cell transplantation (allo-HSCT), luspatercept effectively improved signs of anemia in patients with a variety of hematologic conditions.

Pharmacists also educate, advocate, and ensure patients with heart failure with reduced ejection fraction (HFrEF) and anemia are receiving the appropriate regimens.


Tezepelumab demonstrated efficacy compared with placebo when treating patients with chronic rhinosinusitis with nasal polyps.

The designation was granted after positive results from the phase 1 STOMP-I clinical trial (NCT01915927).