
Glucagon-like peptide-1 (GLP-1) analogs demonstrate potential to address multiple pathological processes in Alzheimer disease, with promising early research suggesting cognitive improvements and potential for future treatment.

Glucagon-like peptide-1 (GLP-1) analogs demonstrate potential to address multiple pathological processes in Alzheimer disease, with promising early research suggesting cognitive improvements and potential for future treatment.

Andrew Charles, MD, outlines why calcitonin-gene-related peptide (CGRP) antagonists are increasingly favored as first-line migraine treatments and how pharmacists play a key role in patient education and support.
A study led by Kayla Johnson, PharmD, BCPS, BCPP found that integrating specialty pharmacists into non-multiple sclerosis (MS) neurology clinics led to significantly enhanced patient care and treatment outcomes.

Beth A. Malow, MD, MS, FAAN, said that pharmacists and health care professionals are at the frontlines of collaborating and raising awareness of climate change�’s impacts.

Malow also emphasized the importance of staying educated on the potential neurological risks that may occur as a result of climate change.

Nilufer Ertekin-Taner outlines how embracing the biological complexity of neurodegenerative diseases can guide the development of precision therapies akin to those used in oncology.
A significant portion of unvaccinated individuals were open to future vaccination.
The trial showed that adding pelabresib to ruxolitinib improved outcomes in myelofibrosis with manageable adverse effects.

Mark Mulvahill, MBA, MSc, discusses the importance of microbial preservation and reference materials in pharmaceutical manufacturing and compounding pharmacies, highlighting their role in contamination control, regulatory compliance, and enhancing microbiological testing reliability.
The poll also showed that social media and general media coverage influenced about 33% of participants to not get vaccinated.
The new designations span both warm autoimmune hemolytic anemia and IgG4-related disease, 2 rare, immune-mediated conditions that burden patients due to a lack of available treatment options.
Compared with the original dose regimen, an antithrombin-based dose regimen of fitusiran was associated with an improved safety profile.
BIIB080 is designed to target microtubule-associated protein tau mRNA, aimed to reduce the production of tau protein.
CAV-1 is a key protein in lipid rafts that helps control cellular processes such as growth, survival, and migration.

Among the 9 surface disinfectants studied, only 2 demonstrated a reduction in viral titer below the limit of detection.

This action makes inebilizumab the first and only FDA-approved treatment for adults with immunoglobulin G4-related disease (IgG4-RD).
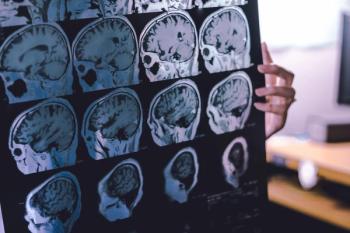
Notably, high levels of high-density lipoprotein cholesterol (HDL-C) were not associated with the risk of dementia following adjustments for triglyceride levels.

Pharmacists can address micronutrient deficiencies, aiding those with type 2 diabetes as they face a higher risk.
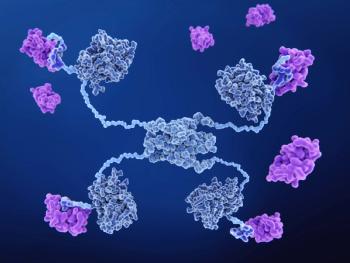
Siremadlin is a novel, investigational, second-generation antagonist that binds to MDM2.

Tocilizumab-bavi received FDA approval in September 2023 as the first biosimilar to tocilizumab for the treatment of various adult and pediatric arthritis conditions.
No manufacturing or safety issues were identified, but the FDA stated the new drug application did not demonstrate efficacy in adequate and well-controlled studies.

Generics play a crucial role in mitigating drug shortages by offering a real alternative for 80% of drugs that are becoming increasingly unavailable in Italy.
Through increased utilization of multi-omic data, health care professionals can revolutionize the treatment paradigm of countless diseases.

The predictive tool may reveal the mechanisms underlying treatment resistance in patients with HER+ breast cancer.
Positive results from an interim analysis of the phase 3 ALIGN study bolstered atrasentan towards receiving accelerated approval for patients with immunoglobulin A nephropathy, a rare condition that can cause kidney failure.
Real-time profiling of T-cell activation states in non-CAR T-cells may help guide treatment decisions.
Pharmacists play a crucial role in educating patients on proper administration and adherence to GLP-1 treatments.

Nicolas Girard, MD, explains the benefits observed in the MARIPOSA clinical trial among patients with epidermal growth factor receptor (EGFR)-mutated non-small cell lung cancer (NSCLC) treated with amivantamab plus lazertinib.
These new phase 3 results indicate the effectiveness of the 2-dose 13-valent pneumococcal conjugate vaccine (PCV13) and 1-dose PCV20 regimens.
The only major change is in the included strain of influenza A/H2N2 virus, which has been updated for both the egg-based and the cell- and recombinant-based vaccines.