
Kyle McCormick, PharmD, owner of Blueberry Pharmacy, discusses the differences between a traditional pharmacy model and a cost-plus pharmacy model.

Kyle McCormick, PharmD, owner of Blueberry Pharmacy, discusses the differences between a traditional pharmacy model and a cost-plus pharmacy model.
Understanding long-term frailty trajectories with kidney function measures could help prevent worse adverse health outcomes in older patients.
Bulevirtide is a synthetic myristoylated peptide derived from the pre-S1 domain of the hepatitis B virus large surface protein.

As pharmacy conglomerates race toward controlling patient journey, community pharmacist role remains murky.
Two recent clinical trials highlight the novel therapy options available to patients in the upfront and relapsed settings.

October is a time to acknowledge the powerful impact pharmacists and pharmacy technicians make on patients and communities.
An integrative efficacy score would incorporate the depth, duration, and prevalence of tumor response.

Tripp Logan, PharmD, vice president at SEMO Rx Pharmacies and Logan & Seiler Inc discusses how pharmacy staff and community health care workers can work together to address social determinants of health.

Bri Morris, PharmD, senior director of Education and the Long-Term Care Division at the National Community Pharmacists Association, discusses the importance of teamwork in the pharmacy.
The history of targeting neuroinflammation is a good example of the challenges to finding a treatment for Alzheimer disease.

Thanks to its diverse patient population and unique culture, studying pharmacy in Hawaii provides interesting experiences with culturally competent care.

Motivational interviewing is an evidence-based method of communicating with patients who are ambivalent or resistant to change for the sake of their health.

The value proposition for point-of-care testing in the pharmacy is extensive, as pharmacies can provide a convenient, accessible, and cost-saving alternative for patients.
Mosunetuzumab showed promising results in complete response rate in patients with follicular lymphoma.

Futibatinib (Lytgobi, Taiho Oncology, Inc) is indicated for adult patients with previously treated, unresectable, locally advanced or metastatic intrahepatic cholangiocarcinoma harboring FGFR2 gene fusions or other rearrangements.

Women Pharmacist Day takes place October 12th, 2022.

Oncology pharmacists participate significantly in conversations, research, and publications of that research.
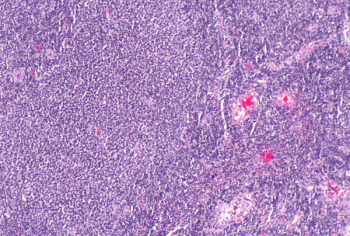
Study results show that intravenous immunoglobulin therapy can help combat this type of PRCA in individuals with anemia.

Teva Pharmaceutical Industries announced that fremanezumab can successfully reduce 2 common migraine comorbid symptoms—anxiety and depression.

Ronna Hauser, PharmD, and Bri Morris, PharmD, both from the National Community Pharmacists Association, discuss long-term care facilities and how they can make an impact on that patient population.

As individuals in a society, all people have implicit, or unconscious, beliefs that they have been socialized to hold; however, health care professionals’ implicit biases can impact patients’ lives.

Pharmacy Times will be spotlighting pharmacists throughout the month of October to recognize their contributions to the health care field.

October is Breast Cancer Awareness Month.

Phoenix-area Walgreens pharmacist Matthew Pruitt earns top honor as 2022 Pharmacist of the Year.
Empagliflozin has been found to lower the risk of death due to cardiovascular events and the number of first and recurrent heart failure hospitalizations.
Study results show that the frequency of adverse events decreased after the patients transitioned from combination therapy to monotherapy.

Indicated for the management of mild, moderate, and severe Alzheimer disease and dementia symptoms, the donepezil transdermal system (Adlarity; Corium) delivers donepezil hydrocholoride through the skin and is recommended at an initial dosage of 5 mg per day.

New research in immunotherapy is exploring vaccines to treat cancer, whether preventative or reactive against current tumors.

Douglas Hoey, CEO of NCPA, discusses NCPA's Annual Convention 2022, which will be held in Kansas City, Missouri from October 1 to October 4.

This month's generic product news features clorazepate dipotassium tablets, exemestane tablets, and paliperidone extended-release tablets.