
When mildly ill, individuals who reflect on the potential consequences of their behavior are less likely to go to holiday gatherings and events.

When mildly ill, individuals who reflect on the potential consequences of their behavior are less likely to go to holiday gatherings and events.

It is important that our national and even state and local professional pharmacy organizations are coming together as a voice for the profession.

Although progress has been made in pre-exposure prophylaxis use, there are still significant barriers for many patients.
Despite a limited understanding about access to oral anticancer drugs, new research finds challenges in patient and clinician decision-making.

Investing in outpatient behavioral health treatment pathways found to be a cost-effective measure to better manage both medical and behavioral conditions.

Although the evidence suggested some deterioration for a few aspects of mental health, the overall findings were mixed with no clear pattern.
The American Heart Association said that yoga, tai-chi, and certain alternative medicines may be beneficial for heart failure, whereas other products may be harmful.

Expert discusses the patient-reported outcomes from the GRIFFIN trial at the final study analysis after all patients completed 1 year of follow-up post maintenance therapy.
Expert discusses updated cohort of patients with longer-term follow up treated in the phase 2 clinical trial of venetoclax added to cladribine plus low-dose araC alternating with azacytidine.
Respiratory syncytial virus- and rhinovirus-induced wheezing illnesses differ from each other at multiple levels.

Patient-controlled analgesia typically consists of opioids or local anesthetics, but it may also include non-opioid analgesics or other medications.

Expert discusses the updated data for a trial cohort after a median follow-up of 27 months.

Adagrasib (Krazati; Mirati Therapeutics, Inc) approved for adults with KRAS-G12C-mutated locally advanced or metastatic non-small cell lung cancer as determined by an FDA-approved test.
Richard T. Maziarz, MD, and a team of investigators assessed average medical costs of allo-HCT throughout a patient’s lifetime and the net monetary savings and value associated with reducing complications.
The FDA clinical reviewer responsible for the approval of this combination therapy discusses the data that led to the approval in May 2022.
It remains a challenge to understand how to stratify risk of liver diseases before they have reached the advanced stage.
Phase 1 clinical trial data show the drug to be active with evidence of clinically meaningful responses and a manageable safety profile.
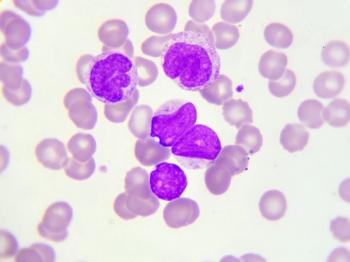
Based on real-world outcomes, there is an unmet need for an effective therapy to be used among patients aged 75 years or older with relapsed/refractory diffuse large B-cell lymphoma.

Regular physical activity helps to facilitate a healthy gut microbiome and lowers inflammation, which were observed in patients independent of body mass index.
Prior research indicates a platelet-based connection between migraine and the patent foramen ovale.
Relatives who attend low-intensity coaching and monitoring meetings increase individuals’ ability to manage the disease.

Following the approval of cabotegravir in 2021, governments and donor agencies in low- and middle-income countries are contemplating replacing oral pre-exposure prophylaxis or augmenting the approach with long-acting treatment.
Long-term data showed no difference in the clinical benefit between the treatments for HER2-negative early breast cancer with homologous recombination deficiency.

Although patients with Richter syndrome often experience poor outcomes, there are many new treatment strategies that have demonstrated a durable response.

Five crucial steps pharmacies can take to enhance patient engagement and lower operational costs.

Herpes zoster and postherpetic neuralgia can cause a significant health burden among adults 65 years of age and older.
Dapagliflozin (Farxiga) found effective in lowering the risk of hospitalization for patients with chronic kidney disease, with or without a diagnosis of type 2 diabetes, and increased the number of days alive and out of the hospital.

Lead compounds may lower the risk of abuse and other adverse effects compared with other drugs currently being evaluated for mental health disorders, such as MDMA.

Study findings may make durvalumab plus chemotherapy a new standard of care option for patients with advanced biliary tract cancer.

A patient with juvenile dermatomyositis experienced aseptic meningitis brought on by intravenous immunoglobulin and recovered fully with no neurological effects.