
Improvements were seen in left ventricular ejection fraction, NYHA Functional Class, and Minnesota Living with Heart Failure Questionnaire at 1-year post-dose.

Improvements were seen in left ventricular ejection fraction, NYHA Functional Class, and Minnesota Living with Heart Failure Questionnaire at 1-year post-dose.
The results suggest that telemedicine could be used to help improve access for other populations that might be stigmatized, beyond hepatitis C virus and opioid use disorder.

Investigators believe that the activation of mast cells drives inflammation and contributes to plaque buildup, causing heart attacks and other damage to the heart.

Artificial intelligence could help detect a trend in medical information for individuals that would help physicians predict an increased risk of sudden cardiac death.

Study investigators found that health care professionals who were assigned male at birth and practiced in the Western region of the United States were more likely to prescribe PrEP to this high-risk population.

Patient sues pharmacy citing communication gaps after prescription was located


The approval makes pembrolizumab the first-line treatment for individuals with this cancer.
The acceptance comes after results from TRANSCEND CLL 004, which is the first trial to demonstrate clinical benefit with a CAR T cell therapy in patients who were previously treated for CLL or SLL.
The approval makes enzalutamide the first and only androgen receptor-signaling inhibitor approved for the treatment of nonmetastatic castration-sensitive prostate cancer with biochemical recurrence at high risk for metastatic.

Capivasertib is indicated for hormone receptor-positive, human epidermal growth factor 2-negative locally advanced or metastatic breast cancer with 1 or more PIK3CA/ALT1/PTEN-alterations.
Not only is the test significant for identifying HIV and TB, but it can be safer for patients living with HIV due to their suppressed immune systems.
The legal and common uses of “interchangeability” continue to be conflated.

In this multi-part series on burnout and the problems currently facing pharmacy, trauma-informed pharmacist Helen Sairany, PharmD, MBA, discusses why burnout is skyrocketing.
In human heart tissues, investigators found that the mechanism associated with heart failure can be reversed and Dicer activity can be restored when AD-9308 was used.

The products include Doxycycline Hyclate Delayed-Release Tablets, Lisdexamfetamine Dimesylate Capsules, Calcium Gluconate in Sodium Chloride Injection, Estradiol Tablets USP

Older treatments and novel options are changing the way obesity is treated and managed
Lisa Deschamps, CEO of AviadoBio, discusses frontotemporal dementia and AVB-101, a one-time therapy designed to stop disease progression by delivering a functional copy of the GRN gene and restoring progranulin levels in the brain.

The approval marks the first authorized at-home sample collection test for sexually transmitted diseases.

In addition to language barriers, pharmacy translation software errors may cause further complications when filling patient prescriptions.

While manganese can sometimes be considered toxic, prenatal exposure may be linked to better memory at adolescence.
Repotrectinib is a tyrosine kinase inhibitor (TKI) that targets ROS1 and is administered as an oral therapy for treatment of locally advanced or metastatic ROS1-positive non–small cell lung cancer.

The study also found that 82% of the surveyed physicians reported not getting labs to help determine treatment options for their patients.

A single infusion of VERVE-101, a gene editing tool, improved both blood PCSK9 protein levels and blood LDL-C levels in 10 individuals with high-risk HeFH.

The RESCUE BT2 trial sheds further light on the agent’s potential.

The products include Crovalimab, Dapagliflozin, Secukinumab, Roflumilast

This interstate agreement can empower pharmacists to respond to large-scale emergencies

Expert discusses changes health care professionals should know about drug coverage and out-of-pocket costs through the IRA
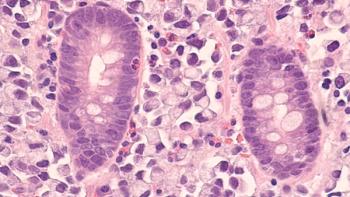
The approval marks the sixth cancer type PD-L1 IHC 22C3 pharmDx has been granted, and the first for GEJ
Taurolidine and heparin (Defencath; CorMedix) is indicated to reduce catheter-related bloodstream infections for adults with kidney failure who are receiving chronic hemodialysis via central venous catheter