
The 11th annual event is scheduled to begin May 5, 2015 at the Wynn Conference Center in Las Vegas.

The 11th annual event is scheduled to begin May 5, 2015 at the Wynn Conference Center in Las Vegas.
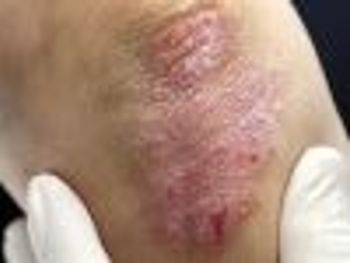
Patients who responded to brodalumab showed improvement in skin condition and joint swelling.

Delegates meet to discuss emerging trends in specialty pharmacy.
Recommendations for co-morbidities identified as an important step for the optimal care of patients.
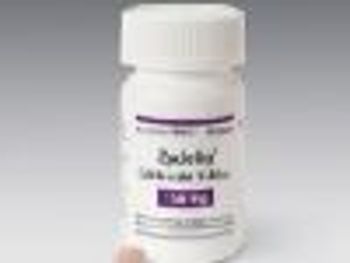
Studies show impressive response rates in patients given Zydelig (idelalisib) for treatment of three types of blood cancer.

NASP/SPAARx merger creates one, unified voice representing the fastest growing segments in the pharmacy and health care industries.

Biologics License Application filed for filgrastim by Sandoz.
Temple University researchers' tool requires further investigation, targets virus cells specifically.

Launch of premium-priced agents powering growth forecast.
Blood test three times more specific in detection of prostate cancer than prostate-specific antigen.

Bill Barre, RPh, vice president of business development at MedImpact Healthcare Systems, discusses key trends in pharmacy benefit management that specialty pharmacies need to be aware of.


Overall annual diagnosis rate dropped 30% from 2002 to 2011, while a significant increase was found among young men who have sex with men.

CEO of Avella Specialty Pharmacy Rebecca M. Shanahan, Esq., discusses how accountable care organizations will impact future trends in specialty pharmacy.

Survey finds an overwhelming number of seniors are unaware of what biosimilars are.
Methotrexate, a traditional disease-modifying antirheumatic drug, may enhance the likelihood of therapeutic persistence with novel biologic agents.

Acquisition includes 33 retail drugstore locations and a specialty pharmacy serving patients with complex or chronic diseases.

ApproveRx is an online platform that streamlines the prior authorization process for pharmacies and prescribers.

Stephen Vogt, PharmD, Chief Executive Officer and President of BioPlus Specialty Pharmacy, discusses the methods BioPlus uses to improve patient medication adherence.

Sunscreen application to infant opossums leads to 10-fold reduction in pre-melanotic lesions.
More than 2 years after ceasing antiretroviral therapy, HIV is again detectable in "Mississippi baby."
Targeted therapies used in conjunction with chemotherapy may offer benefits to patients with triple-negative breast cancer.
A study indicates that BPA-induced cancers in female rats may lead to tumors with evidence of both genetic and epigenetic changes.

Michael Nameth, RPh, MBA, chief executive officer of Aureus Health Services, discusses the growth of his company as a mid-size specialty pharmacy.

Researchers at the Dana-Farber Cancer Institute and Brigham and Women's Hospital have tested a nanomedicine technology in mice formulated to precisely target myeloma cancer cells with a novel bortezomib delivery system.

Study indicates hyperlipidaemia increases risk of breast cancer by 1.64 times.

Executive at Pfizer discusses the various regulatory roadblocks and market challenges for biosimilars in a Q&A with Specialty Pharmacy Times.
Bill Barre, RPh, vice president of business development at MedImpact Healthcare Systems, discusses how MedImpact meets the needs of specialty pharmacies.

People with high levels of sedentary behavior are more susceptible to 3 types of cancer.
Diabetes control worsened for patients with diabetes who received or were receiving androgen deprivation therapy for prostate cancer.