The safety profile was consistent with the profiles associated with each drug as a monotherapy, with no clinically significant drug-drug interactions observed with the combination.

The safety profile was consistent with the profiles associated with each drug as a monotherapy, with no clinically significant drug-drug interactions observed with the combination.
In a presentation at the Community Oncology Alliance 2021 Virtual Conference, Jeff Sharman, MD, summarized the most important developments in care for CLL.
Over the past 18 months, the FDA has approved 4 new regimens for HER2-positive metastatic disease.

A new study published in Nature Communications used artificial intelligence (AI) to identify 3 new multiple sclerosis (MS) subtypes, which may help identify which individuals are more likely to have disease progression, according to the authors.

The researchers found that patients who reduced their calorie intake by 10% or more and adopted a moderate exercise program immediately after their diagnosis had, on average, 70% less chance of having lingering leukemia cells after a month of chemotherapy than those not on the diet-and-exercise regimen.

In the United States, non–small cell lung cancer accounts for 80% to 85% of all lung cancers, with most patients initially diagnosed with advanced or metastatic disease.

The first step toward operationalizing the uptake of biosimilars is having a program-level discussion that not only describes what biosimilars are, but also assesses how they impact the bottom line and could get incorporated into treatment plans.

Opdivo-based combinations show benefit for patients with esophageal cancer who are often diagnosed after their disease has spread and would benefit from new therapeutic options.

Findings from the phase 3 ASCENT trial demonstrate a clinically meaningful 57% reduction in the risk of disease worsening or death for patients receiving sacituzumab govitecan.

Health System Owned Specialty Pharmacy Alliance issues open letter outlining three needed industry actions to improve patient care and outcomes.
In April 2020, colonoscopy screenings declined from 15.1 per 10,000 beneficiaries to 0.9, a 95% difference.

The Pharmacy Times® Pharmacy Focus podcast provides the latest industry news and information, thought-leader insights, clinical updates, patient counseling tools, and innovative solutions for the everyday practice and business of pharmacy.
The researchers analyzed millions of pharmacy transactions by more than 600,000 people in the United States from 2017 to 2019 in order to get a better sense of how vouchers and other point-of-sale co-payment offsets are used.

Isatuximab in combination with carfilzomib and dexamethasone reduced the risk of disease progression or death by 45% versus standard of care alone.

March 31 is International Transgender Day of Visibility, and the Human Rights Campaign (HRC) Foundation and the American Pharmacists Association (APhA) partnered to release a new guide for pharmacies on transgender and gender-diverse inclusion.
Early recognition and treatment, along with a coordinated approach and multidisciplinary team, are important to limit disease progression.

To begin understanding systemic racism and its impact on social determinants of health, researchers said pharmacists must first look inward and examine their own biases and socializations.

Although pediatric patients represent a small portion of total acute myeloid leukemia cases, clinicians said there is a critical need for more effective therapies in this population.

Resilience helps pharmacy personnel cope with mounting pressures and demands that come from stressed patients, tense supervisors, nervous patients, and precarious supply chain balances.
In England, incidence rates of the skin cancer cutaneous malignant melanoma were found to have increased by more than 550% among males and 250% among females since the early 1980s.

In a clinical trial of idecabtagene vicleucel in multiple myeloma, the overall response rate for the efficacy evaluable population was 72%, and 28% of participants achieved a stringent complete response.

In addition to cardiotoxicity from cancer-related treatments, the researchers noted that obesity, cancer, and cardiovascular disease share some common risk factors.
A new study published in The American Journal of Clinical Nutrition found that for colorectal cancer survivors, maintaining a stable body weight may hide a loss of muscle and the development of fatty deposits in their muscles, which resulted in a 40% higher risk of premature death.
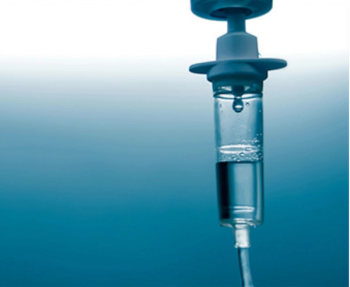
If IVIG is commenced within 2 weeks from the onset of Guillain-Barre syndrome it can hasten recovery as much as plasma exchange, study indicates.

Results from a phase 3 trial investigating the programmed cell death protein 1 inhibitor cemiplimab monotherapy compared to chemotherapy showed an overall survival benefit among patients previously treated with chemotherapy whose cervical cancer is recurrent or metastatic.

Electricity may slow the speed at which breast cancer cells spread through the body, and in some cases may stop them entirely, according to a new study published in Bioelectricity.

Investigators found a statistically significant improvement in both overall survival and progression-free survival for patients who received pembrolizumab with chemotherapy.

Low doses of propylparaben–a chemical preservative found in food, drugs, and cosmetics–can alter pregnancy-related changes in the breast in ways that may lessen the protection against breast cancer normally conveyed by pregnancy hormones.

The IGCS Mentorship and Training Program seeks to provide training and education to regions without formal training programs in gynecologic oncology.

Clinicians have been searching for accurate, reliable, non-invasive diagnostic tools to differentiate early stage, less dangerous, and more treatable stages of the disease from the aggressive, high-grade and likely-to-spread forms.