
Xanomeline and trospium chloride is the first in a new class, offering a new approach with selectively targeting M1 and M4 receptors.

Xanomeline and trospium chloride is the first in a new class, offering a new approach with selectively targeting M1 and M4 receptors.
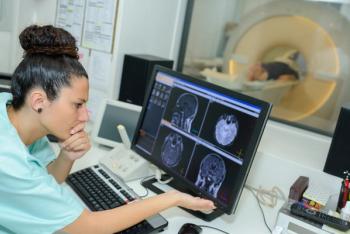
In adolescents, a comprehensive model using behavioral and MRI data had a prediction accuracy of 0.83, which was higher than single data type models.
The discontinuation comes after a review of the medication by the European Medicines Agency which found that “the total number of deaths was higher than anticipated.”

Treatment is typically tailored to the individuals, with HCT being the only curative treatment bone marrow failure, but long-term outcomes are generally poorer due to toxicities.
Additional education can address ambivalence for patients and providers, which can start at the pharmacist level, as they are essential sources of information for patients.

As the end of the DSCSA stabilization period approaches, Josh Bolin from the NABP provides an overview of what to expect.
The authors note this is the first study to assess cerebrovascular endothelial extracellular vesicles within a cognitive condition.

Three of the largest pharmacy benefit managers ––Express Scripts, Optum, and Caremark––are listed in the suit.

The rare disease can result in progressive neurological symptoms and organ complications.

These pharmacies address cost, understanding, and safety concerns through high-touch support and coordination.
In a patient with long-shedding SARS-CoV-2, transfusion-related acute lung injury developed after intravenous immunoglobulin, emphasizing careful treatment considerations.

Eosinophilic granulomatosis with polyangiitis is a rare and immune-mediate vasculitis that can damage multiple organs and be fatal if left untreated.

Eversense 365 (Senseonics) is the first long-lasting continuous glucose monitoring (CGM) system, which includes a 365-day sensor and only 1 insertion on Day 1 of every year.

With the end of the PREP Act provisions approaching, we want to understand how pharmacists feel about their scopes of practice in each state, and what they think about the future of the profession.

Lebrikizumab (Ebglyss; Eli and Lilly Company) is a monthly maintenance injection with proven efficacy in adults and children aged 12 to 18 years.

For biosimilars to overcome these entrenched market dynamics, they must not only compete on price but actively work to build a network of support among providers and patients.

With the introduction of expensive drugs and associated challenges for hospital budgets, pharmacy leaders benefit from strategically managing financial, clinical, and regulatory factors for sustainable care delivery.
The approval marks the first and only twice yearly, health care professional-administrated injection for both forms of multiple sclerosis (MS).
These results build off the PURPOSE 1 trial, contributing to the wealth of evidence surrounding lenacapavir’s positive effects in patients with HIV.

Investigators obtained real-world evidence to evaluate the clinical outcomes of non-medical switching from the infliximab (Remicade; Janssen Immunology) to a biosimilar.

Guselkumab (Tremfya; Johnson & Johnson) is the first and only approved fully human and dual-acting monoclonal antibody blocking interleukin-23 and binding to CD64 receptors.

The company also submitted data from the ongoing phase 2 study, demonstrating the improvements of the investigational drug for symptoms of overall disease severity.

FCS is a severe, rare genetic disease in which patients have extremely high triglyceride levels, typically above 880 mg/dL.
Tocilizumab reduced relapses in 4 patients with myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD).

The FDA assigned a Prescription Drug User Fee Action date is January 31, 2025.

ALZ-801 has now progressed to the phase 3 APOLLOE4 study, designed to evaluate efficacy, safety, and biomarker and imaging effects.

Myasthenia gravis is a chronic autoimmune disease that causes muscle weakness and fatigue, with 4 main treatment options available.

High-cost novel therapies challenge payers and patients, while biosimilars help balance affordability.

An examination of the 340B Drug Pricing Program's original intent, current challenges, and potential resolutions in today's complex health care landscape.

The application includes data from the Vivacity-MG3 study, demonstrating that individuals who received the drug had superior outcomes compared with the standard of care alone.