
This follows the approval of everolimus tablets for patients aged 1 and older in January 2025.

This follows the approval of everolimus tablets for patients aged 1 and older in January 2025.
Through similar targeting of the RdRp enzyme, these treatments could offer groundbreaking new options for patients with COVID-19 if proven effective in real-world trials.
Pharmacists can have informed discussions with patients about the importance of adherence to tuberculosis (TB) treatment regimens, especially for patients with active TB.

Kathy Campbell, PharmD, shares her experiences helping 4 generations of patients.

The authors note that this may cause disruptions in the metabolic stability of lipid processing and storage, impacting the liver and accelerating damaging adaptative responses.

The new indication for semaglutide adds to the long list of conditions that the GLP-1 receptor agonist has been approved for, and provides a new option for high-risk patients with these chronic conditions.
Pharmacist-led interventions significantly improve antibiotic prescribing practices, optimize therapy, and improve patient outcomes.

High-density lipoprotein cholesterol, total cholesterol, and apolipoprotein B did not have significant correlations with chronic obstructive pulmonary disease.
Fractyl Health also reports progress from the REMAIN-1 study evaluating weight management after discontinuing a GLP-1.

Identifying drug-related problems is a vital component of medication therapy management.
The cobas liat system is a closed system that aims to reduce contamination risks and enhances the reliability of results at the point of care.
Antiviral treatments for respiratory viruses such as respiratory syncytial virus (RSV) are crucial, especially with the rise of the "triple-demic."

Although the research focused on Black communities in the United States, there is also evidence linking racism and blood pressure for patients who are Asian or Hispanic.
Approximately 66% of patients received a COVID-19 vaccine in the fall of 2024 and only 26% received a respiratory syncytial virus vaccine.

Remission is a new emphasis and treatment goal for the management of asthma.

Boosting ursodeoxycholic acid (UDCA) levels through dietary supplementation was shown to control tumor growth in mice with liver cancer.

As medication experts, pharmacists can aid in treatment selection and support patients through the process.
Glucose monitoring can help providers and pharmacists optimize medication management for personalized diabetes care.

Inhaling smoke can have acute effect for people with asthma, chronic obstructive pulmonary disease (COPD), or pulmonary fibrosis.

Pharmacists can play an important role in tobacco cessation.

The US health care market faces transformative changes in 2025, as biosimilars, GLP-1s, and new pharmacy economics reshape how benefit managers balance innovation with affordability.

Ruling potentially affects federal ability to regulate pharmacy.
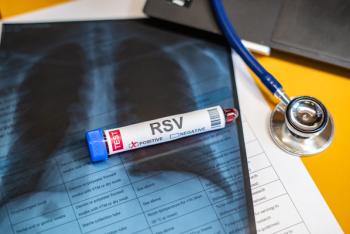
Point-of-care testing implementation could provide valuable data on community respiratory syncytial virus incidence and enhance sentinel surveillance efforts.

Patients recovering from COVID-19 and caregivers reported how some social determinants of health were helpful in their recovery, while others led to difficult social and mental challenges.

The fast-track designation follows the FDA orphan drug designation that was granted for DYNE-101 in September 2023 for the same treatment population.

The Commission aims to provide information for clinical decision making, therapeutic interventions, and public health strategies.

Despite these findings, navacaprant was generally well-tolerated and safe in patients with major depressive disorder.


Memantine/donepezil and everolimus are used to treat dementia of the Alzheimer type and tuberous sclerosis complex-associated subependymal giant cell astrocytoma, respectively.

Patient-centered care involves educating and empowering individuals to take responsibility for their own care.