
Novadoz Pharmaceuticals, who markets the product, has commenced shipping of the drug, available in bottles of 30 tablets.

Novadoz Pharmaceuticals, who markets the product, has commenced shipping of the drug, available in bottles of 30 tablets.

From newsworthy moments to groundbreaking research, these are the most popular articles published on Pharmacy Times® during 2019.

Officials with the FDA have approved Eisai’s lemborexant (Dayvigo) 5 mg and 10 mg for the treatment of adult patients with insomnia, characterized by difficulties with sleep onset and/or sleep maintenance.

Officials with the FDA have approved Intra-Cellular Therapies’ lumateperone (Caplyta) for the treatment of schizophrenia in adults, according to the company.
What classic Christmas movie character fits best with your pharmacy profession?

The applications for the first generics of apixaban (Eliquis, Bristol-Meyers Squibb) tablets were approved to reduce the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation

Ubrogepant is the first drug in the class of oral calcitonin gene-related peptide receptor antagonists approved by the agency for the acute treatment of migraine.
Tazarotene is the first acne treatment to be available in a lotion form, showing strong efficacy with favorable tolerability.

Take care of yourself by providing your body with nutrients that support overall health, your immune system, mood and energy metabolism.
Inflammation from beta blockers is caused by a cellular process known as autophagy.
Research has been conducted to produce a vaccination that gives patients long-lasting protection from influenza.

Jelly of the Month might not have impressed Clark Griswold, but we're sure there's a pharmacist on your gift list that would appreciate one of these items.

Approximately 2 out of 6 study participants tested positive after vaping cannabis that contained 0.39% THC using urine testing methods.

The CDC reported progress with the current status of the 3 strategies within the national initiative, Ending the HIV Epidemic: A Plan for America.

What is causing a patient’s memory loss?

Icosapent ethyl is the first FDA-approved prescription form of fish oil to reduce cardiovascular risk among patients with triglyceride levels as an add-on to maximally tolerated statin therapy.

The Trend Report is a synthesis of multiple research projects exploring risk factors that influence medication adherence, along with background and implications.
A report suggests that addressing financial barriers to antiretroviral therapy adherence may improve levels of viral suppression.
More men than women initiate kidney replacement therapy despite a higher prevalence of chronic kidney disease seen in women.

The FDA has approved the Unidose Liquid System (AptarGroup Inc) as the first and only nasal rescue treatment for acute repetitive seizures in patients with epilepsy.
The US Department of Health and Human Services (HHS) has entered an agreement with Sanofi Pasteur to increase the company's domestic pandemic influenza vaccine production capabilities.

According to Janet Woodcock, MD and director of FDA’s Center for Drug Evaluation and Research, the agency is focused on the drugs used by Americans continuing to meet “strict quality standards.”

The financial consequences of becoming disabled can be devastating if pharmacists lack proper financial planning.

Studying individuals’ genomes can help influence how different medications work for a patient, avoid harmful adverse drug reactions, and allow scientists to develop personalized medicines for patients with a wide range of diseases and disorders.
Top news of the day from across the health care landscape.
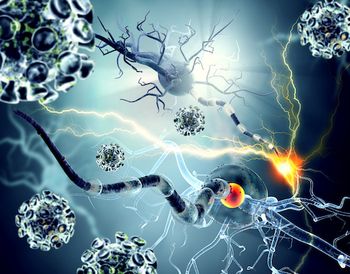
Fingolimod treats relapsing forms of multiple sclerosis in adult patients.

As more vaping cases are reported, patients may desire answers from their trusted providers.

The OTC Guide survey will be open for pharmacists to participate from Nov. 15, 2019, to March 1, 2020.
Louisiana, Nevada, Texas rank highest in flu activity for the month of November.
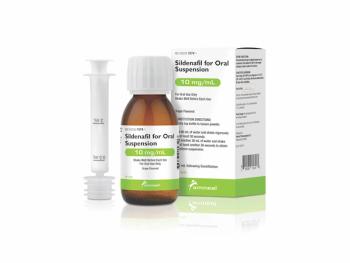
Amneal has begun commercialization activities for the generic versions of sildenafil for oral suspension 10 mg/ml and aminocaproic acid tablets USP, 500 mg.