Omadacycline (Nuzyra) is indicated for the treatment of adult patients with infections caused by susceptible microorganisms—community-acquired bacterial pneumonia and acute bacterial skin and skin structure infections.

Omadacycline (Nuzyra) is indicated for the treatment of adult patients with infections caused by susceptible microorganisms—community-acquired bacterial pneumonia and acute bacterial skin and skin structure infections.

Studies suggest novel immunotherapy may have efficacy as monotherapy and in combination with an anti-PD-1 immune checkpoint inhibitor.
Expanded indication for Rebinyn could allow patients with hemophilia B to participate in physical and social activities.
Compared to atorvastatin, rosuvastatin was associated with higher risks of blood in the urine and kidney failure that requires replacement therapy.

Many companies overlook the power that workplace culture can have on employee wellness and instead, focus relentlessly on financial metrics.
Investigators found that treatment of high-grade squamous intraepithelial lesions in patients with HIV reduced anal cancer incidence by approximately 57%.

Heavy alcohol intake and ALDH2 rs671 polymorphism are among many risk factors associated with increased risk of hepatocellular carcinoma and mortality in patients with hepatitis B virus-related cirrhosis.

Patients with past medical histories of immunoglobulin E-mediated hypersensitivity reactions to metronidazole and other antimicrobial agents may receive safe and effective treatment using desensitization protocols.

Researchers found high levels of childcare stress among female and racial minority health care workers, supporting new programs and wellbeing initiatives.

A majority of long-term care patients who received a fourth vaccination against COVID-19 were found to have increased protection against the omicron variant.

The pandemic saw reductions in the supply of methadone but no disruption to the supply of more easily accessible buprenorphine, though disparities in supply were observed across states.
The findings of the phase 1b/2 CHRYSALIS-2 cohort will be presented at the International Association for the Study of Lung Cancer 2022 World Conference on Lung Cancer.
People who are afraid of shots or feel lightheaded and dizzy while near vaccine injection sites are less likely to get vaccinated against COVID-19.

It’s common practice to recommend heart failure patients follow low sodium diets, but research has shown this type of diet may worsen patient health for certain forms of the condition.

The immune responses to the Omicron variant waned substantially with neutralizing antibody levels decreasing 2.4- to 5.3-fold by 3 months after the booster dose.
Study shows that adult survivors of cancer had a 42% greater risk of CVD than people who did not have the disease.
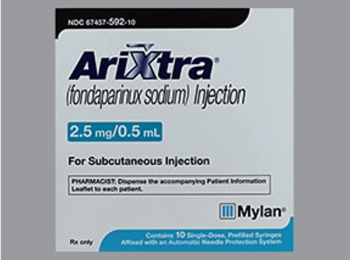
Fondaparinux sodium (Arixtra) is indicated for prophylaxis of deep vein thrombosis (DVT) in patients undergoing hip fracture surgery (including extended prophylaxis), hip replacement surgery, knee replacement surgery, or abdominal surgery.
After a second booster dose, efficacy against COVID-19 Omicron subvariants rose to 80% within the first 6 months, according to results of a study by the CDC.
The drug combination demonstrated a 64.5% confirmed objective response rate in individuals with unresectable locally advanced or metastatic urothelial cancer who are ineligible to receive cisplatin-based chemotherapy.

The FDA is evaluating the use of adagrasib (MRTX849) to treat patients with non-small cell lung cancer harboring a KRAS G12C mutation who have previously received at least 1 systemic therapy.
No differences were found among placenta health indictors, birth weights, or well-being scores between vaccinated and unvaccinated pregnant women, indicating that COVID-19 vaccination is safe for use in pregnant women.

People who performed 2 to 4 times above the recommended amount of moderate physical activity (300-600 minutes/week) saw an overall 26%-31% lower risk of mortality from all causes.
Records from 11 million patients shows that non-alcoholic fatty liver disease could increase the risk of developing heart failure by 50%.

Researchers highlight how a lack of access to medications used to treat multiple sclerosis, migraines, and epilepsy, but not proven safe for pregnancy, threatens child-bearing aged women who live with these conditions.

Belimumab (Benlysta) the first FDA-approved therapy for pediatric lupus nephritis.
Argatroban is indicated for thrombosis in adult patients with heparin-induced thrombocytopenia and as an anticoagulant in adults patients with or at risk for HIT undergoing percutaneous coronary intervention.
Pirtobrutinib is under investigation in clinical trials in patients with CLL/small lymphocytic lymphoma, mantle cell lymphoma, and non-Hodgkin lymphoma.

The number of medical visits carried out via telehealth grew from 840,000 in 2019 to 52.7 million in 2020.

SARS-CoV-2 infection was associated with post-COVID-19 conditions for children, with associated risk factors including length of hospitalization, number of acute symptoms, and older age.

Two pharmacist-based deprescribing models reduced the use and prescription of anticholinergics, a high-risk drug in older adults, providing evidence for the use of pharmacists is practitioners of deprescribing.