
The first buccal film formulation of buprenorphine and naloxone is anticipated to reach retail pharmacies by the end of this week, offering a new treatment option to the more than 2.5 million opioid-dependent patients in the United States.

The first buccal film formulation of buprenorphine and naloxone is anticipated to reach retail pharmacies by the end of this week, offering a new treatment option to the more than 2.5 million opioid-dependent patients in the United States.

The FDA is reviewing a regulatory application to add an asthma indication to Boehringer Ingelheim's chronic obstructive pulmonary disease treatment, tiotropium bromide.

The FDA has expanded its approval of Protein Sciences Corp's Flublok influenza vaccine to cover patients aged 50 years and older, thus permitting its use in all adults.

The FDA's Cardiovascular and Renal Drugs Advisory Committee has recommended the approval of edoxaban (Savaysa) for treating patients with nonvalvular atrial fibrillation, a type of arrhythmia that can potentially cause a stroke.

Xigduo XR combines 2 anti-hyperglycemic agents with complementary mechanisms of action.

Asthmatics with vitamin D deficiency are significantly more susceptible to acute asthma attacks.

The FDA today approved the first vaccine licensed in the United States to prevent invasive serogroup B meningococcal disease in patients aged 10 to 25 years.

Because orally administered morphine is associated with a significantly greater number of adverse events, ibuprofen is the better choice for relieving pain in children with broken bones.

Roughly two-thirds of emergency department admissions for overdoses involve prescription opioid medications.

A drug repackaging company is voluntarily recalling nearly 12,000 boxes of Assured brand naproxen sodium tablets due to a packaging mix-up that left some cartons containing bottles of ibuprofen.

As a result of confusing sensations of breathlessness with asthma symptoms, overweight and obese children tend to report poorer asthma control and overuse rescue medications.

Throughout the month of October, pharmacists are encouraged to share daily facts pertaining to the pharmacy profession via social media in celebration of American Pharmacists Month.

Even though smoking can exacerbate respiratory conditions and limit the efficacy of their treatment, patients often purchase cigarettes while filling prescriptions for asthma or antihypertensive drugs at pharmacies.

The FDA is reviewing a Biologics License Application for the first pediatric combination vaccine designed to help protect against 6 diseases.
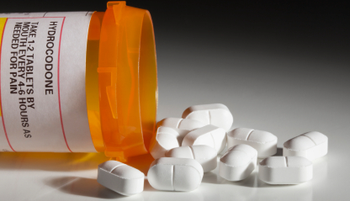
Although pharmacies can legally dispense remaining refills of prescriptions for rescheduled hydrocodone combination products submitted prior to October 6, 2014, over the next 6 months, many drug chains have chosen not to honor those refills.

The FDA today approved pirfenidone (Esbriet) and nintedanib (Ofev) for the treatment of idiopathic pulmonary fibrosis.

The FDA is permitting marketing of a replaceable urinary prosthesis for female adults with impaired detrusor contractility.

Low-dose paroxetine (Brisdelle) treatment does not cause weight gain or sexual dysfunction when used in women with menopausal hot flashes.

Harvoni is comprised of sofosbuvir, a previously approved HCV drug marketed under the brand name Sovaldi, and a new drug called ledipasvir.

The FDA today approved Gilead's Harvoni, the first combination pill to treat chronic hepatitis C virus genotype 1 infection.

The FDA today approved the first drug-coated balloon for reopening arteries in the thigh and knee that are narrowed or blocked due to peripheral artery disease.

The drugmaker will no longer pursue FDA approval of its dual daclatasvir and asunaprevir treatment for patients with hepatitis C virus genotype 1b.

Pharmacy benefit services integrated within health insurance plans result in improved medical outcomes and cost savings compared with services administered separately from the insurer.

All drugs containing the opioid hydrocodone have been reclassified as Schedule II controlled substances, prohibiting pharmacies from recognizing refills and phoned-in prescriptions for those medications.

Tabalumab failed to establish efficacy compared with current standard of care therapy in 2 pivotal late-stage trials.

By generating thousands of tweets using the hashtag #Pharmacist, pharmacy organizations and pharmacists around the world have made their impact on patients and the health care industry known to the global Twitter community.

Pharmacists and pharmacy organizations around the world are taking to Twitter to spread the word about their impact on patients, communities, and the health care industry.

The FDA has approved the intravitreal implant, Iluvien 0.19 mg, as the first long-term treatment for diabetic macular edema.

In celebration of American Pharmacists Month, pharmacists are encouraged to spread the word about their work throughout the month of October.

The dexamethasone intravitreal implant, Ozurdex, is now indicated to treat all patients with diabetic macular edema.