
The single-dose, prefilled autoinjector for tocilizumab (Actemra) offers an additional option for patients with rheumatoid arthritis, giant cell arteritis, and 2 forms of juvenile arthritis.

The single-dose, prefilled autoinjector for tocilizumab (Actemra) offers an additional option for patients with rheumatoid arthritis, giant cell arteritis, and 2 forms of juvenile arthritis.

Tailoring hepatitis C treatments to shorten regimen length can reduce costs and ease burden for patients with limited insurance benefits.

With this approval, venetoclax (Venclexta) offers a new treatment option for individuals who are unable to tolerate standard intensive chemotherapy.

With this approval, venetoclax (Venclexta) offers a new treatment option for individuals who are unable to tolerate standard intensive chemotherapy.

Glasdegib (Daurismo) is the first and only Hedgehog pathway inhibitor approved for the treatment of acute myeloid leukemia.
Tivozanib (Fotivda) improved progression-free survival and reduced risk of death in patients with highly refractory advanced or metastatic disease.

Officials with the FDA have approved emapalumab (Gamifant) for the treatment of pediatric and adult patients with primary hemophagocytic lymphohistiocytosis (HLH), making it the first FDA-approved drug specifically indicated for this disease.

Emapalumab (Gamifant) is the first treatment specifically approved for primary hemophagocytic lymphohistiocytosis, a rare and life-threatening immune disease.

Study identifies potential biomarkers that could provide a quicker path to diagnosis of amyotrophic lateral sclerosis.

Eli Lilly has submitted a New Drug Application (NDA) to the FDA for lasmiditan for the acute treatment of migraine with or without aura in adults.

Lasmiditan’s mechanism is distinct from other therapies and, if approved, represents significant innovation in the acute treatment of migraine.
A new study shows high prevalence of atopic dermatitis among adults, illustrating the need for more available treatment options.
In a session held at the National Association of Specialty Pharmacy Annual Meeting and Educational Conference, Kara E Cardinale, MPA, provided an update on regulatory actions in the health care space by the Centers for Medicare and Medicaid Services (CMS) and Department of Health and Human Services.
In a presentation held at the 2018 National Association of Specialty Pharmacy Annual Meeting and Educational Conference, Doug Long, MBA, provided insight into the growth of specialty, citing several areas to watch over the next few years.
With oncology agents now flooding the specialty drug pipeline, it is important to keep up with the latest developments and look ahead at innovative therapies on the horizon.

Study evaluates the association between body mass index in late adolescence and the incidence of pancreatic cancer in adulthood.

The FDA granted the designation to brentuximab vedotin (Adcetris) for this indication in combination with cyclophosphamide, doxorubicin, and prednisone.
Express Scripts announced the impending launch of a novel formulary designed to ease out-of-pocket cost burden for members by allowing health plans to cover lower list price drug products.

The new formulary will allow health plans to cover lower list price products, such as new authorized alternatives to brand-name medications.
The FDA accepted a supplemental New Drug Application for olaparib’s (Lynparza) new indication for first-line maintenance treatment of advanced ovarian cancer.

The FDA accepted a supplemental New Drug Application for olaparib’s (Lynparza) new indication for first-line maintenance treatment of advanced ovarian cancer.
A new report published in the CDC Morbidity and Mortality Weekly Report (MMWR) has linked cases of acute hepatitis A virus (HAV) infections from certain states to person-to-person transmission from drug use or homelessness.
The epidemiology of hepatitis A has shifted from point-source outbreaks to outbreaks caused by person-to-person transmission.

If approved, avatrombopag (Doptelet) would also be indicated for the treatment of chronic immune thrombocytopenia in patients who have had insufficient response to a previous therapy.
Although malignant melanoma incidence has stabilized or declined in women, melanoma-associated mortality rates have risen among men throughout the world.

An up-to-date analysis of melanoma mortality rates show that deaths among men are rising, but stabilizing or decreasing in women.

Skin cancer remains the most common form of cancer in the United States, with more than 65,000 cases of melanoma estimated in 2011, according to the CDC.
Seattle Genetics submitted an application for the expanded use of brentuximab vedotin (Adcetris) in combination with a chemotherapy regimen for patients with peripheral T-cell lymphoma.
Prescriptions for hormonal therapy medications increased in states that expanded Medicaid, according to a recent analysis.
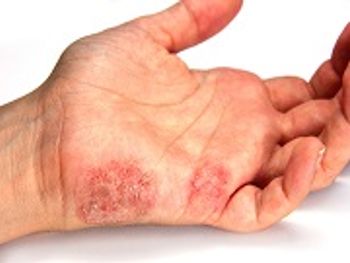
Officials with the FDA have granted Priority Review to dupilumab (Dupixent, Sanofi) for moderate-to-severe atopic dermatitis in adolescent patients aged 12 to 17 years.