
Bausch + Lomb has expanded the power range of its Trulign Toric intraocular lens (IOL), providing eye care providers more options for cataract patients.

Bausch + Lomb has expanded the power range of its Trulign Toric intraocular lens (IOL), providing eye care providers more options for cataract patients.

Reslizumab targets a key cytokine involved in elevating levels of blood eosinophils.

Drug shows improvement in skin thickening with active treatment.

The FDA is currently reviewing Teva's reslizumab treatment for adult and adolescent asthmatics with symptoms inadequately controlled by inhaled corticosteroid (ICS) regimens.
The FDA has granted Breakthrough Therapy Designation to Roche's tocilizumab (Actemra/RoActemra) for the treatment of systemic sclerosis.
First treatment approved for ASMD/Niemann-Pick disease type B.

The FDA has granted breakthrough therapy designation to Genzyme's olipudase alfa enzyme replacement treatment for nonneurological manifestations of acid sphingomyelinase deficiency.
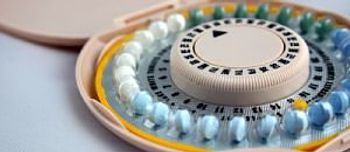
Mylan has introduced a generic form of Teva's Seasonique extended-cycle birth control pill to the US market.

Teva has introduced norethindrone acetate-ethinyl estradiol/ferrous fumarate (Junel Fe 24) tablets, a generic alternative to Amneal's Lomedia 24 Fe oral contraceptive, to the US market.