
This month's OTC product new features, eye drops, hemp oil for dogs, and daily supplement packs.

This month's OTC product new features, eye drops, hemp oil for dogs, and daily supplement packs.

Colleges of pharmacy are essential to providing equal access and quality of care to underserved communities.

Pharmacists can offer medication adherence and management counseling and help patients with chronic disease treatments.

Depression can make people who have recovered from COVID-19 feel less enthusiastic in their daily lifestyle.
Conditions such as high blood pressure, diabetes, and obesity may have a significant impact on the likelihood of developing cognitive decline, dementia, and Alzheimer disease.
Letermovir has been approved by the FDA to prevent severe CMV in patients who are asymptomatic and undergoing allogeneic hematopoietic stem cell transplants.
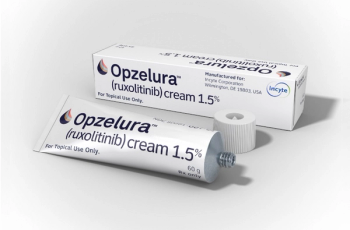
Ruxolitinib (Opzelura) is indicated for the topical short-term and non-continuous chronic treatment of mild to moderate atopic dermatitis.

Kaopectate treats diarrhea, heartburn, nausea, and upset stomach.
Three in 5 say it is hard to get kids to eat well-balanced diets, because of not eating enough fruits and vegetables, pickiness, and other reasons.

FDA-approved Disulfiram can safely reduce anxiety levels in rodents, analysis from the Tokyo University of Sciences and other Japan institutes finds.

Psychedelic medicines like psilocybin work to re-wire the brain, potentially addressing the root cause of eating disorders rather than the symptoms.

On this week's episode, Suzy is joined by Michelle Henry, MD, from Skin & Aesthetic Surgery of Manhattan, a board-certified dermatologist located in New York.

The first cases were identified in October 2021 at a children’s hospital in Alabama that admitted 5 children with significant liver injury, including some with acute liver failure.

USP 800 was a necessary step to maximize safety, but it also recommended many institutions reevaluate their facilities, equipment, processes, and staffing capacity, potentially costing millions of dollars.

How accurate do our favorite movies and television shows represent pharmacology and other processes in the pharmacy profession?

The booster vaccine candidate includes mutations found in the Beta variant of concern, several of which have been persistent in more recent variants such as the Omicron variant.

By attending more rural pharmacy programs, students said they had a unique perspective and experiences working with rural populations.

The results lead to a possible new therapeutic approach to improve immunotherapy outcomes, including combinations with vitamin E, as well as directly targeting SHP1 in dendritic cells.

DuoBody, for treatment of relapsed/refractory large B-cell lymphoma, demonstrates an overall response rate of 63.1%.

New analysis demonstrates that physical activity improves cognitive health, thus staving off dementia.

Laly Havern, PharmD, MS, BCACP, director in specialty health solutions leading in oncology and fertility at Walgreens, discusses the company's Fertility Awareness Campaign that is being launched as part of National Infertility Awareness Week.
In a live virtual symposium at the 2021 ASHP Midyear Clinical Meeting, 2 experts teamed up to highlight the place in therapy and management of oral oncolytics in follicular and marginal zone lymphoma.
32 public colleges with combined enrollment of more than 700,000 students must comply with law by January 2023.

Elaine Jaffe, MD, National Institutes of Health (NIH) distinguished investigator at the National Cancer Institute at NIH, offers guidance for female medical researchers who are faced with a declining number of female colleagues in the workforce.

Kelan Thomas, PharmD, MS, discussed potential adverse effects and drug-drug interactions that pharmacists should be aware of with psychedelic medicines.

Pilocarpine (Vuity) is a cholinergic muscarinic receptor agonist indicated for the treatment of presbyopia in adults.

PinkEye Relief are sterile eye drops that temporarily relieve burning, swelling, and crusting.
The CIC is a combination of Novavax’s COVID-19 vaccine, NVX-CoV2373, and its quadrivalent influenza vaccine candidate.
Each time a pharmacist immunizes a patient against one of the 27 infections for which a safe and effective vaccine exists, the profession continues to protect communities against diseases that wreaked havoc on generations.

The application is based on positive results from the ASCEND phase 3 clinical trial, assessing the efficacy and safety of daprodustat for the treatment of anemia from chronic kidney disease.