
Tracy Russell, senior director, State Government Affairs at CoverMyMeds, discusses state and federal legislation that may be impacting the future of specialty pharmacies and the patient care they deliver.

Tracy Russell, senior director, State Government Affairs at CoverMyMeds, discusses state and federal legislation that may be impacting the future of specialty pharmacies and the patient care they deliver.

Imposter syndrome is a phenomenon that affects self-confidence, significantly limiting the effectiveness of professionals affected by it.
An international phase 1/2 clinical trial is ongoing for the treatment for individuals with relapsed or refectory acute myeloid leukemia with FLT3 mutation.

First phase 3 trial in this setting shows improved overall survival rates with an immunotherapy added to chemotherapy over the standard-of-care chemotherapy alone, company says.

Jelmyto is an alkylating drug indicated for the treatment of adult patients with low-grade upper tract urothelial cancer.
Palbociclib is indicated for adults in combination with an aromatase inhibitor as initial endocrine-based therapy in postmenopausal women or in men.

The drug combination achieved a median overall survival of more than 5 1/2 years in the first-line setting for postmenopausal women with HR+/HER2- aBC1, the company says.
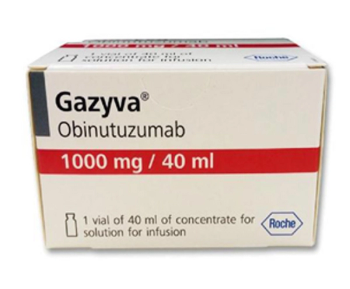
Obinutuzumab is a CD20-directed cytolytic antibody used to treat certain types of blood cancer.

High adherence to treatment is a key first step toward better patient outcomes, including sustained disease control or remission.

Sneha Sharma, director, specialty clinical solutions at Magellan Health, discusses some trends in the rare disease space that she recommends pharmacists keep an eye on this year.
Pharmacists are well suited to help patients overcome the barriers that prevent access to therapy regimens.

Amy Ware, director, specialty clinical solutions at Magellan Health, discusses what it means to be future focused as a pharmacist in the specialty pharmacy field.

Trastuzumab deruxtecan (Enhertu) approved for adult patients with unresectable or metastatic HER2-positive breast cancer who were previously administered an anti–HER2-based regimen.

Jayne Hornung, chief clinical officer – pharmacy, Managed Markets Insight & Technology, discusses her presentation on potential causes of the slow uptake in digital therapeutics among payers.

Tracy Russell, senior director, State Government Affairs at CoverMyMeds, discusses her presentation at Asembia Summit 2022 on legislation aligned with the movement to expand the pharmacist’s role on the care team.

Regardless of the ability to access and dispense products, pharmacists are responsible for getting patients the best care.

Pharmacists can play a more active role in educating patients on tools and resources available to them, enrolling patients in programs, gathering subjective and objective data.

Ray Tancredi, divisional vice president of Specialty Pharmacy, Development & Brand Rx/Vaccine Purchasing at Walgreens, addresses his presentation on the specialty pharmaceutical pipeline, and specifically what approvals pharmacists should look for on the horizon.

Sonia T. Oskouei, PharmD, vice president of biosimilars at Cardinal Health, discussed how pharmacists can impact biosimilar adoption.

Jayson Slotnik, JD, MPH, partner at Health Policy Strategies, discusses his hopes for the future of value-based contracting and the ideal model for pharmacy’s involvement.

Trials results show darolutamide, docetaxel, and androgen deprivation therapy (ADT) reduced the risk of death by 32.5% versus docetaxel/ADT in patients with metastatic hormone-sensitive prostate cancer.

Patient data are essential to providing coordinator care, but more data also creates heightened risks.

Prescription medications are vital to optimal patient outcomes, but they are also becoming increasingly expensive.

Ray Tancredi, divisional vice president of Specialty Pharmacy, Development & Brand Rx/Vaccine Purchasing at Walgreens, discusses some of the more significant drugs for specialty pharmacy in the FDA pipeline this year.

Digital medicine is the use of technology to measure or intervene in certain health conditions and is overseen by the FDA, unlike digital health apps or technologies.

A panel of experts at the Asembia 2022 Specialty Pharmacy Summit discussed some of the obstacles in providing medications approved under Emergency Use Authorization.
Discovery has the potential to also benefit treatment choices, investigators from Georgetown Lombardi Comprehensive Cancer Center say.
Vosevi is a fixed-dose combination product containing sofosbuvir, velpatasvir, and voxilaprevir for the re-treatment of adults with chronic hepatitis C.

Tracy Russell, senior director of State Government Affairs at CoverMyMeds, discusses how the role of the pharmacist in the specialty pharmacy space has expanded during the COVID-19 pandemic.
Tizlelizumab is a unique anti-PD-1 monoclonal antibody that is being analyzed in 14 pivotal clinical trials across an array of solid tumors.