
Diplomat Specialty Pharmacy CEO Phil Hagerman was recently invited to participate in a forum on fostering greater opportunity, innovation, and job creation in today's economy.

Diplomat Specialty Pharmacy CEO Phil Hagerman was recently invited to participate in a forum on fostering greater opportunity, innovation, and job creation in today's economy.

On June 20th, 2012, the longest day of the year, the Alzheimer's Association is asking participants to run, walk, bike, or engage in some kind of endurance activity for 16 consecutive hours to honor those facing Alzheimer's disease.

The FDA recently tested samples of compounded hydroxyprogesterone caproate (the active ingredient in Makena) to measure their potency and purity.

SXC Health Solutions Corp announced that it has been awarded Specialty Pharmacy Accreditation from URAC, a Washington, DC-based health care accrediting organization that establishes quality standards for the health care industry.
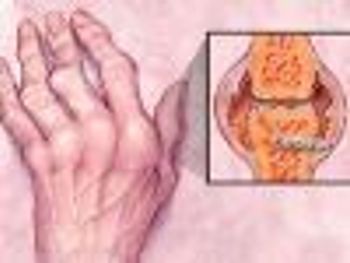
Phase 3 study findings showed that patients with active moderate to severe rheumatoid arthritis (RA) who received an investigational intravenous (IV) formulation of the anti-tumor necrosis factor (TNF)-alpha therapy Simponi (golimumab) demonstrated significant improvements in signs, symptoms, and disease activity.
The FDA will take an additional 3 months to review the drug application for Truvada as a preventive HIV treatment to account for updated safety materials submitted by Gilead.

Argus Health Systems, Inc, a leading transparent pharmacy benefits administrator, recently announced that it has established a Preferred Specialty Program with Amber Pharmacy to provide expanded network options.
The FDA approved Genentech, Inc's pertuzumab injection (Perjeta) for use in combination with trastuzumab and docetaxel for the treatment of patients with HER2-positive metastatic breast cancer who have not received prior anti-HER2 therapy or chemotherapy for metastatic disease.

Diplomat Specialty Pharmacy, the nation's largest, privately owned specialty pharmacy, announced that pharmacy executive, Gary W. Kadlec, has joined the company and will be serving as president.

Pharmaceutical Strategies Group, LLC, recently announced the immediate availability of a suite of specialty pharmacy management services featuring their RxDiagnostic for Specialty service.
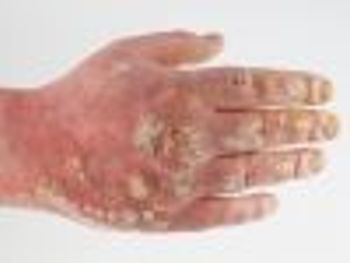
New efficacy and safety data from the Phase 3 PHOENIX 1 study, 1 of 2 pivotal registration trials, showed that maintenance treatment with Stelara (ustekinumab) for up to 5 years resulted in consistent, significant clinical response in adults with moderate to severe plaque psoriasis.

MedPro Rx receives accreditation from the Accreditation Commission for Health Care, Inc, in the areas of Infusion Pharmacy Services, Infusion Nursing Services, and Specialty Pharmacy Services.

Bristol-Myers Squibb's investigational drug facilitates improved immune response in lung cancer, kidney cancer, and melanoma patients, according to a study presented at the American Society of Clinical Oncology (ASCO).

Beckie Fenrick, PharmD, MBA, discusses emerging trends in specialty pharmacy including companion diagnostics and personalized care.
Girls who received mantle radiation for childhood cancer were found to be at elevated risk for secondary breast cancer later in life.
Afatinib shows promise as a treatment for patients with advanced lung adenocarcinomas who tested positive for EGFR mutations.

A new single agent called dabrafenib for the treatment of metastatic melanoma appears to significantly slow progression of the disease, according to reports from the American Society of Clinical Oncology (ASCO) meeting.

Employers are concerned with worker health behaviors and want to control spending on expensive specialty medications, according to a new survey released by Express Scripts.

Testing this sample of the population could help identify more than 800,000 new cases of hepatitis C and address the largely preventable consequences of the condition.

Cardinal Health Specialty Solutions today launched PathWare Decision Transaction Solutions, a new technology system that makes it easier for payors and physicians to work together to implement programs that improve the quality and cost of caring for patients.

Vital Care, Inc recently announced that its network of franchised home infusion pharmacies has expanded to Indiana.

Michael Einodshofer, RPh, MBA, Director of Utilization Management, Walgreens Specialty Pharmacy, addresses site of care optimization as a strategy for health plans to lower costs on specialty drugs.
Researchers will receive $7.9 million over the next 5 years to test the effects of intranasal insulin therapy on patients with mild cognitive impairment and mild to moderate Alzheimer's disease.

In this AJMC video, Surya Singh, MD, vice president, medical benefit management, CVS Caremark, discusses a variety of approaches used to stem the rise of costs associated with oncology.

The plan marks the creation of a landmark national strategy to increase public awareness of Alzheimer's disease.

Patients with certain preexisting heart conditions, history of stroke, or those who are taking antiarrhythmic medications are being advised to use the oral MS agent Gilenya cautiously.
An FDA advisory panel has recommended Truvada as a pre-exposure prophylaxis for people at risk of contracting HIV.
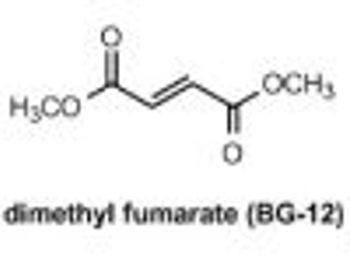
Biogen Idec recently announced that United States and European Union regulatory authorities have accepted the company's marketing applications for the review of BG-12 (dimethyl fumarate), an oral therapeutic candidate for the treatment of multiple sclerosis (MS).
The committee voted 8-2 to recommend approval of the investigational agent tofacitinib, and an FDA decision could come as early as August.
The Food and Drug Administration is alerting health care professionals and patients about injuries and death associated with the use of an experimental procedure sometimes called "liberation therapy" or the "liberation procedure" to treat chronic cerebrospinal venous insufficiency (CCSVI).