
Avella Specialty Pharmacy today announced it has been identified as one of a select group of specialty pharmacies to supply Tarceva (erlotinib).

Avella Specialty Pharmacy today announced it has been identified as one of a select group of specialty pharmacies to supply Tarceva (erlotinib).

Patient engagement, financial backing, and personalized medicine-3 of the most pressing issues in drug development and regulatory affairs-will be discussed at length at the DIA 2013 49th Annual Meeting, June 23-27 in Boston.

The court's decision makes it more likely that generic versions of medications will be made available before their patents expire and will likely lead to reduced drug costs for consumers.
New therapies advance the treatment of this hematologic cancer.

Michael Einodshofer, director, utilization management, Walgreens, describes how contractual methodologies and cost differences across various sites of service can influence how a specialty drug is reimbursed under the medical benefit.

Consumer Watchdog's latest lawsuit questions the legality of another mandatory mail-order program for specialty pharmaceuticals.

The Supreme Court today ruled that because DNA is a "product of nature," it cannot be protected by a patent-but complementary DNA made synthetically in a lab would be eligible for patent protection.
The FDA today approved denosumab (Xgeva injection, for subcutaneous use) for the treatment of adults and skeletally mature adolescents with giant cell tumor of bone that is unresectable or where surgical resection is likely to result in severe morbidity.

A study published online in JAMA Psychiatry found that hospitalization for an infection or an autoimmune disease increased the risk of a subsequent mood disorder diagnosis.

At the Association for Health Insurance Plan's (AHIP) conference this Thursday, medical directors from Cardinal Health Specialty Solutions and Aetna will provide expert insight into how collaboration between payers and physicians can directly lead to better cancer care that is more effective and costs less.

CVS Caremark announced today that it has been awarded Specialty Pharmacy accreditation from URAC, a Washington, DC-based health care accrediting organization that establishes quality standards for the health care industry.

Debbie Stern, RPh, president of Rxperts, explains why a high entry cost for a specialty drug may dissuade a patient from filling and staying adherent to his or her prescription.

The new process takes into account the efficacy, risk, cost, and societal benefit of a given specialty drug in determining whether it should be added to the hospital's formulary.

The Biotechnology Industry Organization (BIO) and Oregon Bioscience Association (Oregon Bio) commend Oregon Governor John Kitzhaber for signing legislation designed to address the regulatory issues related to the interchangeability of biological medicines.

Adam Fein describes the way in which the sequester will affect physician reimbursement for specialty drugs.
Illinois is now the 13th state to avoid enacting legislation that would slow patient access to biosimilars, which are new, affordable versions of costly brand biologic medicines that treat cancer, immune disorders and other complex diseases.

Prime Therapeutics, a leading pharmacy benefit manager, announced today that its specialty pharmacy, Prime Therapeutics Specialty Pharmacy, has received full accreditation from URAC.

The last day of ASCO is today! Check out these social media updates to see what has been popular at the meeting.

The Specialty Pharmacy Association of America (SPAARx) announced it is has agreed to participate in an alliance with BusinessOne Technologies (BusinessOne) wherein BusinessOne will provide access to its national/regional market access data and data integration technology.

Ongoing shortages of common oncology chemotherapies have compelled physicians to substitute more expensive drugs, delay or suspend clinical trials, or even skip doses of chemotherapy, according to survey results presented at ASCO.

Michael Einodshofer of Walgreens discusses the challenges associated with moving specialty into retail in this video shot at PBMI's Drug Benefit Conference 2013.

The letters ask 15 health insurance companies in New York to adopt a "Specialty Prescription Drug Fulfillment Hardship Exception Criteria" so that patients who want to get their specialty prescriptions at a community pharmacy may do so without penalty.
Bevacizumab is the first biologic, targeted treatment for cervical cancer proven to significantly improve survival, according to Phase III research released ahead of the plenary session of the 49th Annual Meeting of the American Society of Clinical Oncology.

The addition of GM-CSF to ipilimumab treatment for patients with metastatic melanoma was found to decrease mortality risk by 35%, according to a study presented today at ASCO.
An investigational monoclonal antibody called daratumumab has received breakthrough therapy designation from the FDA for the treatment of patients with double refractory multiple myeloma, according to a statement from the drug's manufacturer, Janssen Biotech.

Armada Health Care (Armada), the nation's largest group purchasing and channel management organization for the specialty pharmacy market, is pleased to appoint Mike Baldzicki as Executive Vice President of Industry Relations & Advocacy.

Pharmacy team helps patients better understand importance of adherence while on complex medication regimens.
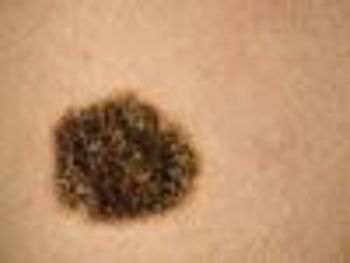
The FDA today approved two new drugs, Tafinlar (dabrafenib) and Mekinist (trametinib), for patients with metastatic or unresectable melanoma.

Manufacturers can use a variety of alternative distribution methods to reduce drug waste and prepare for the cost structure changes associated with health care reform.

Adam J. Fein describes how the 340B program is being exploited by many hospital systems due to a lack of oversight by government agencies.