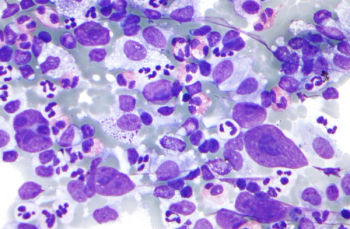
Belantamab Mafodotin Combination Shows Durable Efficacy in Relapsed or Refractory Multiple Myeloma
Belantamab mafodotin plus lenalidomide and dexamethasone was found tolerable with no new safety signals and enhanced response rates in patients with multiple myeloma.