About the Author
Ryan Fernholz is a PharmD Candidate in the North Dakota State University School of Pharmacy class of 2024.
Preceptor: Krysta Larson, PharmD
Promising data have emerged suggesting a significant weight loss benefit for patients using GLP-1 agonists and GLP-1/GIP dual agonists.
Background
According to National Institutes of Health data from 2018, approximately 1 in 3 individuals in the United States are either obese or morbidly obese.1 Estimates of the medical cost of adult obesity in the United States range from $147 billion to nearly $210 billion per year.2 Over the past few years, promising data have emerged suggesting a significant weight loss benefit for patients using glucagon-like peptide (GLP)-1 agonists and GLP-1/glucose-dependent insulinotropic polypeptide (GIP) dual agonists.3,4,5 There are currently 3 FDA-approved medications for the indication of weight loss, with the most recent being tirzepatide (Zepbound; Lilly), a GLP-1/GIP dual agonist.
Image credit: Fernanda | stock.adobe.com
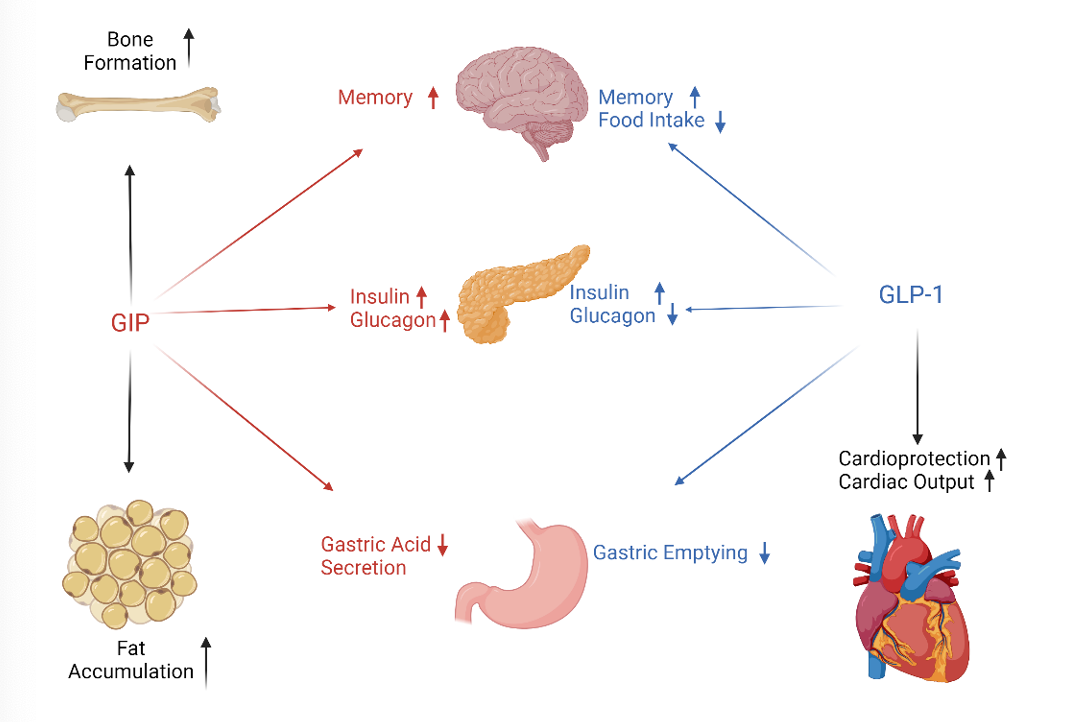
Mechanism of GLP-1 and GIP for Weight Loss
GLP-1 is a naturally occurring peptide in the gut that regulates insulin release. As GLP-1 expression is increased, the body will release more insulin. This will also lead to a delay in gastric emptying and a decrease in appetite.6 GIP, another nutrient-stimulated hormone, regulates energy balance through cell-surface receptor signaling in the brain and adipose tissue. GIP works by promoting dietary lipid storage and acts in the central nervous system to lower food intake.7
Effects of GLP-1 and GIP | Created with biorender.com, derived from Yabe, et al.8
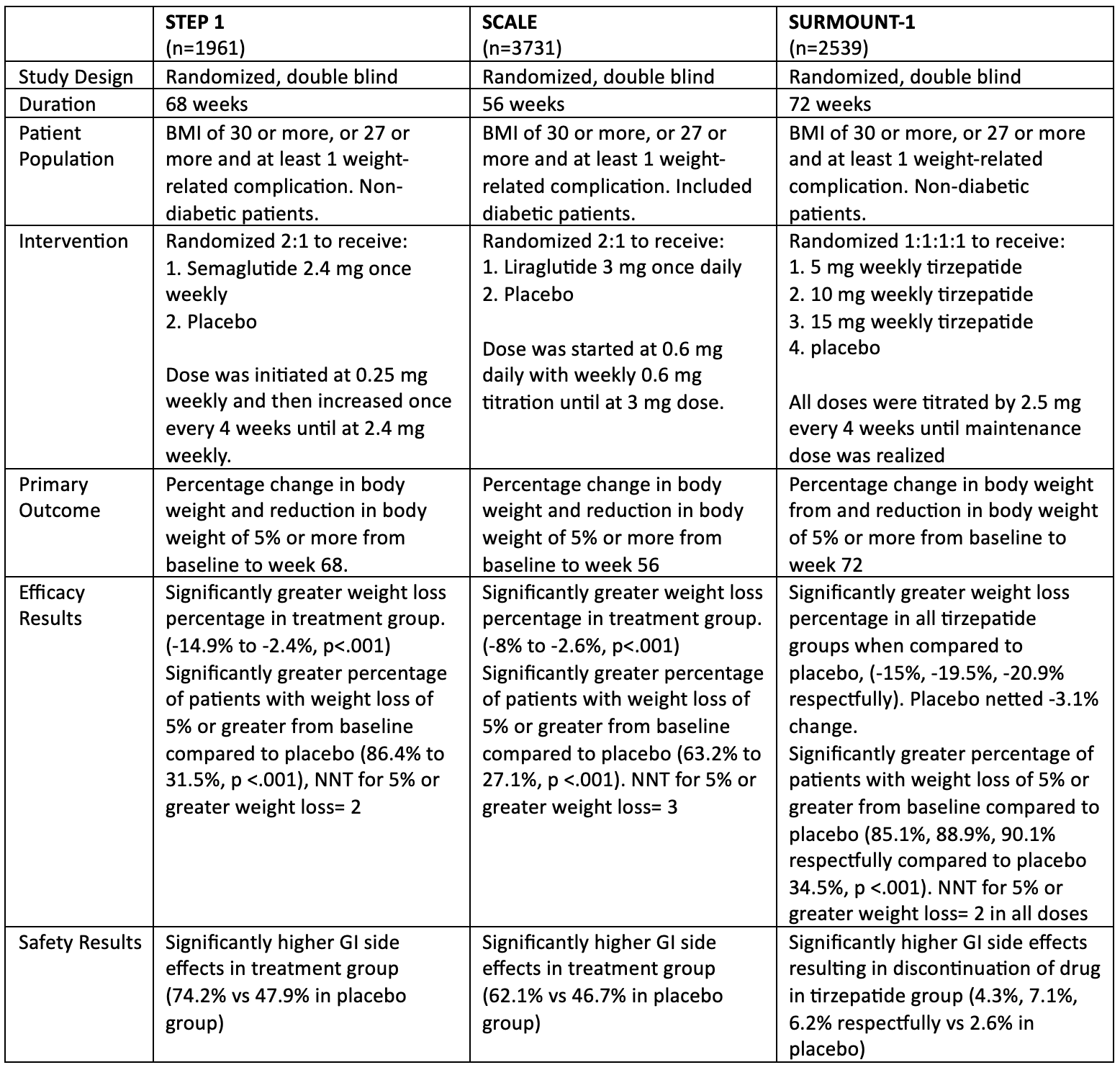
Evidence for GLP-1/GIP Agonists
There is currently only 1 FDA-approved GLP-1/GIP agonist for weight loss (tirzepatide). A 72-week trial for this medication showed a significant weight loss benefit compared to placebo that was dose dependant.5 A significantly higher amount of people in the treatment groups in this trial dropped out due to gastrointestinal side (GI) effects, similar to GLP-1 agonists in previous studies.3,4 Comparisons between tirzepatide and the GLP-1 agonists liraglutide (Saxenda; Novo Nordisk) and semaglutide (Wegovy; Novo Nordisk) can be found in Table 1.
There are currently no completed studies directly comparing GLP-1 agonists with GLP-1/GIP dual agonists. However, there is an ongoing trial looking at semaglutide vs tirzepatide for weight loss in patients without type 2 diabetes mellitus.9
Table 1. Comparisons Between Studies of Tirzepatide, Liraglutide, and Semaglutide
Cost Analysis and Impact on Health Care
According to a study conducted from 2000 through 2016, patients who were obese had on average double the amount of medical expenditures than those who were normal weight ($5010 vs $2504).10 Currently, Medicare does not cover any portion of weight loss medications without a diabetes diagnosis and allows states to decide whether they will cover weight loss medications. Many states still do not cover these medications, but Minnesota has liraglutide and semaglutide currently on their preferred drug list. Wisconsin, on the other hand, requires a prior authorization for any weight loss medication.
Many commercial insurers, however, have started to cover these medications at least partially without a diabetes diagnosis. Below is a comparison of the retail cost of tirzepatide versus annual medical expenditures of individuals with obesity:
Ryan Fernholz is a PharmD Candidate in the North Dakota State University School of Pharmacy class of 2024.
Preceptor: Krysta Larson, PharmD
A study was conducted to compare the cost efficiency of tirzepatide vs semaglutide when used for weight loss. The researchers compared results from the STEP-1 and SURMOUNT-1 trials using a cost needed to treat analysis. They used pricing data from October 2022 using GoodRx. It was found that by taking the weekly cost of semaglutide at $336 in the STEP-1 trial for semaglutide, patients would spend $17,495 over a single year. The weekly cost of tirzepatide in the SURMOUNT-1 trial was $243 using GoodRx prices, making the cost $12,658 over 1 year. They also used these results to analyze cost per 1% body fat reduction. Tirzepatide had a cost of $985 (95% CI: $908-$1075) per 1% body fat reduction whereas semaglutide had a cost per 1% body fat reduction of $1845 (95% CI: $1707-$1989).11